Topic: Atomic Concepts
Atomic Concepts
Which statement describes the earliest model of the atom?
(1) An atom is an indivisible hard sphere.
(2) An atom has a small, dense nucleus.
(3) Electrons are negative particles in an atom.
(4) Electrons in an atom have wave-like properties.
The results of the gold foil experiment led to the conclusion that an atom is
(1) mostly empty space and has a small, negatively charged nucleus
(2) mostly empty space and has a small, positively charged nucleus
(3) a hard sphere and has a large, negatively charged nucleus
(4) a hard sphere and has a large, positively charged nucleus
The discovery of the electron as a subatomic particle was a result of
(1) collision theory
(2) kinetic molecular theory
(3) the gold-foil experiment
(4) experiments with cathode ray tubes
Given a list of atomic model descriptions:
A: electron shells outside a central nucleus B: hard, indivisible sphere C: mostly empty space
Which list of atomic model descriptions represents the order of historical development from the earliest to most recent?
(1) A, B, C
(2) A, C, B
(3) B, C, A
(4) B, A, C
Four statements about the development of the atomic model are shown below.
- A: Electrons have wavelike properties. B: Atoms have small, negatively charged particles. C: The center of an atom is a small, dense nucleus. D: Atoms are hard, indivisible spheres.
Which order of statements represents the historical development of the atomic model?
(1) C → D → A → B
(2) C → D → B → A
(3) D → B → A → C
(4) D → B → C → A
Which conclusion was drawn from the results of the gold foil experiment?
(1) An atom is electrically neutral.
(2) An atom is mostly empty space.
(3) The nucleus of an atom is negatively charged.
(4) The electrons in an atom are located in specific shells.
As a result of the gold foil experiment, it was concluded that an atom
(1) contains protons, neutrons, and electrons
(2) contains a small, dense nucleus
(3) has positrons and orbitals
(4) is a hard, indivisible sphere
Which particles are found in the nucleus of an argon atom?
(1) protons and electrons
(2) positrons and neutrons
(3) protons and neutrons
(4) positrons and electrons
The part of an atom that has an overall positive charge is called
(1) an electron
(2) the nucleus
(3) the first shell
(4) the valence shell
Which statement describes the charge and location of an electron in an atom?
(1) An electron has a positive charge and is located outside the nucleus.
(2) An electron has a positive charge and is located in the nucleus.
(3) An electron has a negative charge and is located outside the nucleus.
(4) An electron has a negative charge and is located in the nucleus.
Which phrase describes an Al atom?
(1) a negatively charged nucleus, surrounded by negatively charged electrons
(2) a negatively charged nucleus, surrounded by positively charged electrons
(3) a positively charged nucleus, surrounded by negatively charged electrons
(4) a positively charged nucleus, surrounded by positively charged electrons
Which statement describes the structure of an atom?
(1) The nucleus contains positively charged electrons.
(2) The nucleus contains negatively charged protons.
(3) The nucleus has a positive charge and is surrounded by negatively charged electrons.
(4) The nucleus has a negative charge and is surrounded by positively charged electrons.
A student compares some models of the atom. These models are listed in the table below in order of development from top to bottom.
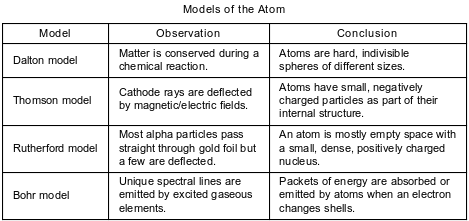
State one way in which the Bohr model agrees with the Thomson model.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Atoms have electrons.
• Atoms have small, negatively charged particles.
• Both models show an internal structure.
• Atoms are neutral.
The diagrams below represent four different atomic nuclei.
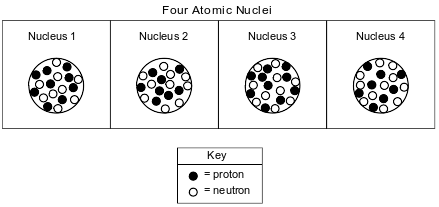
Identify the nucleus above that is found in an atom that has a stable valence electron configuration.
Allow 1 credit. Acceptable responses include, but are not limited to:
• nucleus 3
Elements with an atomic number greater than 92 can be artificially produced in nuclear reactions by bombarding a naturally occurring nuclide with a different nuclide. One of these elements is roentgenium, Rg. The equation below represents a nuclear reaction that produces Rg-272.
![]()
State the location and the total charge of the protons in a Ni-64 atom.
Allow 1 credit for a correct combination of the location and the total charge of the protons.
• Acceptable responses include, but are not limited to:
• Location of protons:
• in the nucleus
• the small, dense center of an atom
• center of an atom
• Total charge of protons:
• +28
• 28+
• 28
