Topic: Atomic Models
Atomic Models
Which statement describes the earliest model of the atom?
(1) An atom is an indivisible hard sphere.
(2) An atom has a small, dense nucleus.
(3) Electrons are negative particles in an atom.
(4) Electrons in an atom have wave-like properties.
The results of the gold foil experiment led to the conclusion that an atom is
(1) mostly empty space and has a small, negatively charged nucleus
(2) mostly empty space and has a small, positively charged nucleus
(3) a hard sphere and has a large, negatively charged nucleus
(4) a hard sphere and has a large, positively charged nucleus
The discovery of the electron as a subatomic particle was a result of
(1) collision theory
(2) kinetic molecular theory
(3) the gold-foil experiment
(4) experiments with cathode ray tubes
Given a list of atomic model descriptions:
A: electron shells outside a central nucleus B: hard, indivisible sphere C: mostly empty space
Which list of atomic model descriptions represents the order of historical development from the earliest to most recent?
(1) A, B, C
(2) A, C, B
(3) B, C, A
(4) B, A, C
Four statements about the development of the atomic model are shown below.
- A: Electrons have wavelike properties. B: Atoms have small, negatively charged particles. C: The center of an atom is a small, dense nucleus. D: Atoms are hard, indivisible spheres.
Which order of statements represents the historical development of the atomic model?
(1) C → D → A → B
(2) C → D → B → A
(3) D → B → A → C
(4) D → B → C → A
Which conclusion was drawn from the results of the gold foil experiment?
(1) An atom is electrically neutral.
(2) An atom is mostly empty space.
(3) The nucleus of an atom is negatively charged.
(4) The electrons in an atom are located in specific shells.
As a result of the gold foil experiment, it was concluded that an atom
(1) contains protons, neutrons, and electrons
(2) contains a small, dense nucleus
(3) has positrons and orbitals
(4) is a hard, indivisible sphere
Which statement describes a concept included in the wave-mechanical model of the atom?
(1) Protons, neutrons, and electrons are located in the nucleus.
(2) Electrons orbit the nucleus in shells at fi xed distances.
(3) Atoms are hard, indivisible spheres.
(4) Electrons are located in regions called orbitals.
An orbital is a region in an atom where there is a high probability of finding
(1) an alpha particle
(2) an electron
(3) a neutron
(4) a positron
According to the wave-mechanical model of the atom, electrons are located in
(1) orbitals
(2) circular paths
(3) a small, dense nucleus
(4) a hard, indivisible sphere
According to the wave-mechanical model, an orbital is defined as the most probable location of
(1) a proton
(2) a neutron
(3) a positron
(4) an electron
Which statement describes the location of protons and neutrons in an atom of helium?
(1) Protons and neutrons are in the nucleus.
(2) Protons and neutrons are outside the nucleus.
(3) Protons are outside the nucleus, and neutrons are in the nucleus.
(4) Protons are in the nucleus, and neutrons are outside the nucleus.
Which term is defined as the region in an atom where an electron is most likely to be located?
(1) nucleus
(2) orbital
(3) quanta
(4) spectra
Which term identifies the most probable location of an electron in the wave-mechanical model of the atom?
(1) anode
(2) orbital
(3) nucleus
(4) cathode
A student compares some models of the atom. These models are listed in the table below in order of development from top to bottom.
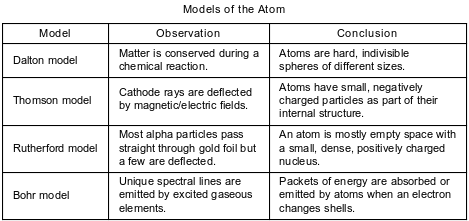
State one way in which the Bohr model agrees with the Thomson model.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Atoms have electrons.
• Atoms have small, negatively charged particles.
• Both models show an internal structure.
• Atoms are neutral.
