Topic: Bonding Tendency Of Elements
Bonding Tendency Of Elements
Which compound has covalent bonds?
(1) H2O
(2) Li2O
(3) Na2O
(4) K2O
Ice, H2O(s), is classified as
(1) an ionic compound
(2) a molecular compound
(3) a homogeneous mixture
(4) a heterogeneous mixture
Which compound contains both ionic and covalent bonds?
(1) KI
(2) CaCl2
(3) CH2Br2
(4) NaCN
Which elements can react to produce a molecular compound?
(1) calcium and chlorine
(2) hydrogen and sulfur
(3) lithium and fluorine
(4) magnesium and oxygen
Which element reacts with oxygen to form ionic bonds?
(1) calcium
(2) hydrogen
(3) chlorine
(4) nitrogen
What is formed when two atoms of bromine bond together?
(1) a monatomic molecule
(2) a diatomic molecule
(3) a heterogeneous mixture
(4) a homogeneous mixture
In the compound KHSO4, there is an ionic bond between the
(1) KH+ and SO42− ions + and O2− ions
(2) KHSO3
(3) K+ and HS− ions − ions
(4) K+ and HSO4
Some compounds of silver are listed with their chemical formulas in the table below.
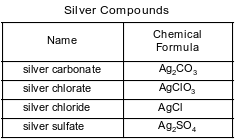
Explain, in terms of element classification, why silver chloride is an ionic compound.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The reaction between a metal and a nonmetal can produce an ionic compound.
• Silver is a metal and chlorine is a nonmetal.
The elements in Group 17 are called halogens. The word “halogen” is derived from Greek and means “salt former.”
Identify the type of chemical bond that forms when potassium reacts with bromine to form a salt.
Allow 1 credit. Acceptable responses include, but are not limited to:
• ionic bond
• ionic
Explain, in terms of element classification, why K2O is an ionic compound.
Allow 1 credit. Acceptable responses include, but are not limited to:
• A metal reacts with a nonmetal to produce an ionic compound.
• Potassium is a metal and oxygen is a nonmetal.
In a laboratory investigation, ammonium chloride was dissolved in water. Laboratory procedures and corresponding observations made by a student during the investigation are shown in the table below.

Identify two types of bonds in the solute.
Allow 1 credit. Acceptable responses include, but are not limited to:
• ionic bonds and polar covalent bonds
• covalent and ionic
One type of soap is produced when ethyl stearate and sodium hydroxide react. The soap produced by this reaction is called sodium stearate. The other product of the reaction is ethanol. This reaction is represented by the balanced equation below.
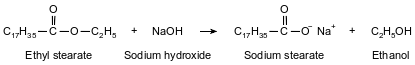
Identify the two types of bonds in the compound sodium stearate.
Allow 1 credit. Acceptable responses include, but are not limited to:
• covalent bonds and ionic bonds
• polar and nonpolar
• single and double
John Dalton, an early scientist, sketched the structure of compounds using his own symbols for the elements known at the time. Dalton’s symbols for four elements and his drawing of potassium aluminum sulfate are represented by the diagram below.
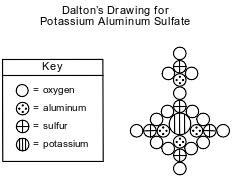
Today, it is known that the chemical formula for potassium aluminum sulfate is KAl(SO4)2•12H2O. It is a hydrated compound because water molecules are included within its crystal structure. There are 12 moles of H2O for every 1 mole of KAl(SO4)2. The compound contains two different positive ions. The gram-formula mass of KAl(SO4)2•12H2O is 474 grams per mole.
Identify one positive ion in the hydrated compound. Your response must include both the chemical symbol and charge of the ion.
Allow 1 credit. Acceptable responses include, but are not limited to:
• K+
• Al3+
During a fireworks display, salts are heated to very high temperatures. Ions in the salts absorb energy and become excited. Spectacular colors are produced as energy is emitted from the ions in the form of light.
The color of the emitted light is characteristic of the metal ion in each salt. For example, the lithium ion in lithium carbonate, Li2CO3, produces a deep-red color. The strontium ion in strontium carbonate, SrCO3, produces a bright-red color. Similarly, calcium chloride is used for orange light, sodium chloride for yellow light, and barium chloride for green light.
Identify the two types of chemical bonds found in the salt used to produce a deep-red color.
Allow 1 credit. Acceptable responses include, but are not limited to:
• ionic bonds and polar covalent bonds
• covalent and ionic
