Topic: Compounds
Compounds
What is the chemical formula for sodium oxalate?
(1) NaO
(2) Na2O
(3) NaC2O4
(4) Na2C2O4
What is the chemical name of the compound NH4SCN?
(1) ammonium thiocyanate
(2) ammonium cyanide
(3) nitrogen hydrogen cyanide
(4) nitrogen hydrogen sulfate
Which statement explains why NaBr is classifi ed as a compound?
(1) Na and Br are chemically combined in a fixed proportion.
(2) Na and Br are both nonmetals.
(3) NaBr is a solid at 298 K and standard pressure.
(4) NaBr dissolves in H2O at 298 K.
What is the formula for iron(II) oxide?
(1) FeO
(2) FeO2
(3) Fe2O
(4) Fe2O3
Which substance can be broken down by chemical means?
(1) ammonia
(2) aluminum
(3) antimony
(4) argon
Which formula represents ammonium nitrate?
(1) NH4NO3
(2) NH4NO2
(3) NH4(NO3)2
(4) NH4(NO2)2
What is represented by the chemical formula PbCl2(s)?
(1) a substance
(2) a solution
(3) a homogeneous mixture
(4) a heterogeneous mixture
What is the chemical formula for ammonium sulfide?
(1) (NH4)2S
(2) (NH4)2SO3
(3) (NH4)2SO4
(4) (NH4)2S2O3
What is the chemical formula of titanium(II) oxide?
(1) TiO
(2) Ti2O
(3) TiO2
(4) Ti2O3
What is the chemical formula for sodium sulfate?
(1) Na2SO4
(2) Na2SO3
(3) NaSO4
(4) NaSO3
A compound is a substance composed of two or more elements that are
(1) physically mixed in a fixed proportion
(2) physically mixed in a variable proportion
(3) chemically combined in a fixed proportion
(4) chemically combined in a variable proportion
During a laboratory activity, appropriate safety equipment was used and safety procedures were followed. A laboratory technician heated a sample of solid KClO3 in a crucible to determine the percent composition by mass of oxygen in the compound. The unbalanced equation and the data for the decomposition of solid KClO3 are shown below.
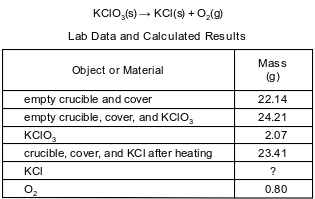
Write a chemical name for the compound that decomposed.
Allow 1 credit for potassium chlorate.
Thermal energy is absorbed as chemical reactions occur during the process of baking muffins. The batter for muffins often contains baking soda, NaHCO3(s), which decomposes as the muffins are baked in an oven at 200.°C. The balanced equation below represents this reaction, which releases CO2(g) and causes the muffins to rise as they bake. The H2O(ℓ) is released into the air of the oven as it becomes a vapor.
2NaHCO3(s) + heat → Na2CO3(s) + H2O(ℓ) + CO2(g)
Based on Table E, identify the polyatomic ion in the solid product of the reaction.
Allow 1 credit. Acceptable responses include, but are not limited to:
• carbonate ion
• carbonate
• CO32−
A sample of seawater is analyzed. The table below gives the concentration of some ions in the sample.
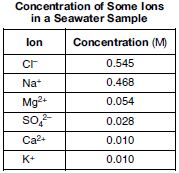
Write a chemical formula of one compound formed by the combination of K+ ions with one of these ions as water completely evaporates from the seawater sample.
Allow 1 credit. Acceptable responses include, but are not limited to:
• KCl
• K2SO4
Baking soda, NaHCO3, can be commercially produced during a series of chemical reactions called the Solvay process. In this process, NH3(aq), NaCl(aq), and other chemicals are used to produce NaHCO3(s) and NH4Cl(aq).
To reduce production costs, NH3(aq) is recovered from NH4Cl(aq) through a different series of reactions. This series of reactions can be summarized by the overall reaction represented by the unbalanced equation below.
NH4Cl(aq) + CaO(s) → NH3(aq) + H2O(ℓ) + CaCl2(aq)
Write a chemical name for baking soda.
Allow 1 credit. Acceptable responses include, but are not limited to:
• sodium hydrogen carbonate
• sodium bicarbonate
• sodium acid carbonate
• monosodium carbonate
• bicarbonate of soda
