Topic: Concentration Of A Solution
Concentration Of A Solution
What is the molarity of 2.0 liters of an aqueous solution that contains 0.50 mole of potassium iodide, KI?
(1) 1.0 M
(2) 2.0 M
(3) 0.25 M
(4) 0.50 M
What is the molarity of 0.50 liter of an aqueous solution that contains 0.20 mole of NaOH (gram-formula mass = 40. g/mol)?
(1) 0.10 M
(2) 0.20 M
(3) 2.5 M
(4) 0.40 M
A solution contains 25 grams of KNO3 dissolved in 200. grams of H2O. Which numerical setup can be used to calculate the percent by mass of KNO3 in this solution?
(1) ![]()
(2) ![]()
(3) ![]()
(4) ![]()
A solution is prepared using 0.125 g of glucose, C6H12O6, in enough water to make 250. g of total solution. The concentration of this solution, expressed in parts per million, is
(1) 5.00 × 101 ppm
(2) 5.00 × 102 ppm
(3) 5.00 × 103 ppm
(4) 5.00 × 104 ppm
What is the concentration of an aqueous solution that contains 1.5 moles of NaCl in 500. milliliters of this solution?
(1) 0.30 M
(2) 0.75 M
(3) 3.0 M
(4) 7.5 M
In a laboratory investigation, an HCl(aq) solution with a pH value of 2 is used to determine the molarity of a KOH(aq) solution. A 7.5-milliliter sample of the KOH(aq) is exactly neutralized by 15.0 milliliters of the 0.010 M HCl(aq). During this laboratory activity, appropriate safety equipment is used and safety procedures are followed.
Show a numerical setup for calculating the molarity of the KOH solution.
Allow 1 credit. Acceptable responses include, but are not limited to:
• ![]()
A bottled water label lists the ions dissolved in the water. The table below lists the mass of some ions dissolved in a 500.-gram sample of the bottled water.
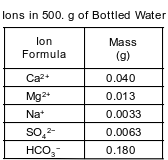
Show a numerical setup for calculating the parts per million of the Na+ ions in the 500.-gram sample of the bottled water.
Allow 1 credit. Acceptable responses include, but are not limited to:
• 
In a laboratory activity, a student titrates a 20.0-milliliter sample of HCl(aq) using 0.025 M NaOH(aq). In one of the titration trials, 17.6 milliliters of the base solution exactly neutralizes the acid sample.
Show a numerical setup for calculating the concentration of the hydrochloric acid using the titration data.
Allow 1 credit. Acceptable responses include, but are not limited to:
• MA(20.0 mL) = (0.025 M)(17.6 mL)
• ![]()
A student constructs an electrochemical cell. A diagram of the operating cell and the unbalanced ionic equation representing the reaction occurring in the cell are shown below.
The blue color of the solution in the copper half-cell indicates the presence of Cu2+ ions. The student observes that the blue color becomes less intense as the cell operates.
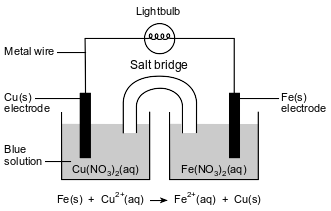
State one inference that the student can make about the concentration of the Cu2+ ions based on the change in intensity of the color of the Cu(NO3)2(aq) solution as the cell operates.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The concentration of the Cu2+ ions decreases.
• There are fewer copper ions in the solution.
In a laboratory activity, each of four different masses of KNO3(s) is placed in a separate test tube that contains 10.0 grams of H2O at 25°C.
When each sample is first placed in the water, the temperature of the mixture decreases. The mixture in each test tube is then stirred while it is heated in a hot water bath until all of the KNO3(s) is dissolved. The contents of each test tube are then cooled to the temperature at which KNO3 crystals first reappear. The procedure is repeated until the recrystallization temperatures for each mixture are consistent, as shown in the table below.
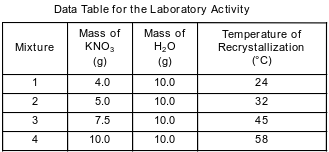
Determine the percent by mass concentration of KNO3 in mixture 2 after heating.
Allow 1 credit for 33% or any value from 33% to 33.3¯¯% inclusive.
A solution is made by dissolving 70.0 grams of KNO3(s) in 100. grams of water at 50.°C and standard pressure.
Show a numerical setup for calculating the percent by mass of KNO3 in the solution.
Allow 1 credit. Acceptable responses include, but are not limited to:
• ![]()
A solution of ethylene glycol and water can be used as the coolant in an engine-cooling system. The ethylene glycol concentration in a coolant solution is often given as percent by volume. For example, 100. mL of a coolant solution that is 40.% ethylene glycol by volume contains 40. mL of ethylene glycol diluted with enough water to produce a total volume of 100. mL. The graph below shows the freezing point of coolants that have different ethylene glycol concentrations.

One engine-cooling system has a volume of 6400 mL. Determine the volume of ethylene glycol in the completely filled engine-cooling system when the concentration of ethylene glycol is 50.% by volume.
Allow 1 credit. Acceptable responses include, but are not limited to:
During a titration, 10.00 mL of acetic acid, HC2H3O2(aq), is completely neutralized by adding 12.50 mL of 0.64 M sodium hydroxide, NaOH(aq).
Explain why it is better to use data from multiple trials to determine the molarity of acetic acid, rather than data from a single trial.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Multiple trials may improve the precision of results.
• Each trial may involve errors either above or below the acceptable value. Therefore, an average value may be more accurate.
• Results can be shown to be reproducible.
• Multiple trials help cancel random errors.
In an investigation, aqueous solutions are prepared by completely dissolving a different amount of NaCl(s) in each of four beakers containing 100.00 grams of H2O(ℓ) at room temperature. Each solution is heated and the temperature at which boiling occurred is measured. The data are recorded in the table below.
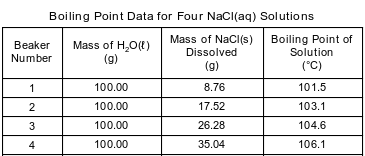
Show a numerical setup for calculating the percent by mass of NaCl in the solution in beaker 4.
Allow 1 credit. Acceptable responses include, but are not limited to:
• 
During the winter months, icy roads pose a threat to motorists and can lead to accidents. A mixture of sand and sodium chloride, NaCl, can be spread on roads to make winter driving safer.
One New York town requires that a mixture of sand and salt used on residential roads should contain 25% or less of NaCl by mass. A 10.0-gram sample of a mixture of sand and NaCl was analyzed and found to contain 3.3 grams of NaCl.
Explain, in terms of composition by mass, why the mixture from which the analyzed sample was taken should not be used on residential roads of the town.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The sample is greater than 25% NaCl by mass.
• The ratio by mass of sand to NaCl in the sample is 2 to 1.
• The mass of the salt is too great.
