Topic: Formulas
Formulas
Which term represents the fi xed proportion of elements in a compound?
(1) atomic mass
(2) molar mass
(3) chemical formula
(4) density formula
Which two terms represent types of chemical formulas?
(1) mechanical and structural
(2) mechanical and thermal
(3) molecular and structural
(4) molecular and thermal
Which two terms represent types of chemical formulas?
(1) fission and fusion
(2) oxidation and reduction
(3) empirical and structural
(4) endothermic and exothermic
Which two terms represent types of chemical formulas?
(1) empirical and molecular
(2) polar and nonpolar
(3) synthesis and decomposition
(4) saturated and concentrated
The empirical formula for butene is
(1) CH2
(2) C2H4
(3) C4H6
(4) C4H8
Which formula is an empirical formula?
(1) N2O4
(2) NH3
(3) C3H6
(4) P4O10
A metal worker uses a cutting torch that operates by reacting acetylene gas, C2H2(g), with oxygen gas, O2(g), as shown in the unbalanced equation below.
C2H2(g) + O2(g) → CO2(g) + H2O(g) + heat
Write the empirical formula for acetylene.
Allow 1 credit for CH or HC.
Ethyl ethanoate is used as a solvent for varnishes and in the manufacture of artifi cial leather. The formula below represents a molecule of ethyl ethanoate.
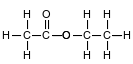
Write the empirical formula for this compound.
Allow 1 credit for C2H4O. The order of the elements may vary.
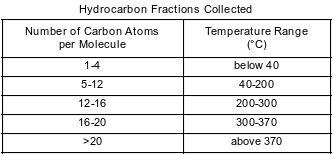
Crude oil, primarily a mixture of hydrocarbons, is separated into useful components in a fractionating tower. At the bottom of the tower, the crude oil is heated to about 400°C. The gases formed rise and cool. Most of the gases condense and are collected as liquid fractions. The table below shows the temperature ranges for collecting various hydrocarbon fractions.
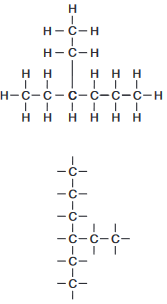
Draw a structural formula for 3-ethylhexane.
Allow 1 credit.
• Examples of 1-credit responses:
• 
Fatty acids, a class of compounds found in living things, are organic acids with long hydrocarbon chains. Linoleic acid, an unsaturated fatty acid, is essential for human skin flexibility and smoothness. The formula below represents a molecule of linoleic acid.
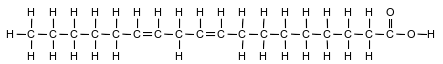
Write the molecular formula of linoleic acid.
Allow 1 credit for C18H32O2. The order of the elements may vary.
Tetrachloroethene, C2Cl4, is a solvent used in many dry cleaning processes.
Write the empirical formula for tetrachloroethene.
Allow 1 credit for CCl2. The order of the elements may vary.
The table below contains selected information about chlorine and two compounds containing chlorine. One piece of information is missing for each of the substances in the table.
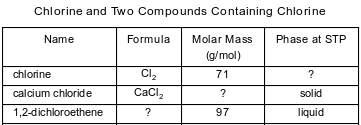
The liquid compound has an empirical formula of CHCl. Write the molecular formula for this compound.
Allow 1 credit for C2H2Cl2. The order of the elements may vary.
Fruit growers in Florida protect oranges when the temperature is near freezing by spraying water on them. It is the freezing of the water that protects the oranges from frost damage. When H2O(ℓ) at 0°C changes to H2O(s) at 0°C, heat energy is released. This energy helps to prevent the temperature inside the orange from dropping below freezing, which could damage the fruit. After harvesting, oranges can be exposed to ethene gas, C2H4, to improve their color.
Write the empirical formula for ethene.
Allow 1 credit for CH2. The order of the elements may vary.
What is the empirical formula for C6H12?
Allow 1 credit for CH2. The order of the elements can vary.
During photosynthesis, plants use carbon dioxide, water, and light energy to produce glucose, C6H12O6, and oxygen. The reaction for photosynthesis is represented by the balanced equation below.
6CO2 + 6H2O + light energy → C6H12O6 + 6O2
Write the empirical formula for glucose.
Allow 1 credit for CH2O. The order of the elements may vary.
