Topic: Half Reaction
Half Reaction
Which process can be represented by a half- reaction equation?
(1) distillation
(2) oxidation
(3) sublimation
(4) vaporization
Which half-reaction equation represents reduction?
(1) Cu → Cu2+ + 2e−
(2) Cu2+ + 2e− → Cu
(3) Ag + e− → Ag+
(4) Ag+ → Ag + e−
Given the equation representing a reaction:
Cd + NiO2 + 2H2O → Cd(OH)2 + Ni(OH)2
Which half-reaction equation represents the oxidation in the reaction?
(1) Ni4+ + 2e− → Ni2+
(2) Ni4+ → Ni2+ + 2e−
(3) Cd → Cd2+ + 2e−
(4) Cd + 2e− → Cd2+
Which equation represents a reduction half- reaction?
(1) Fe → Fe3+ + 3e–
(2) Fe + 3e− → Fe3+
(3) Fe3+ → Fe + 3e–
(4) Fe3+ + 3e− → Fe
Which type of equation can represent the oxidation occurring in a reaction?
(1) a double-replacement reaction equation
(2) a half-reaction equation
(3) a neutralization reaction equation
(4) a transmutation reaction equation
The diagram and ionic equation below represent an operating voltaic cell.
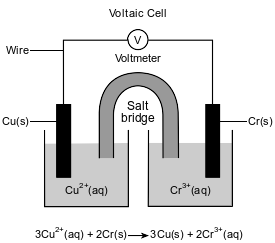
Write a balanced equation for the half-reaction that occurs in the copper half-cell when the cell operates.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Cu2+(aq) + 2e− → Cu(s)
• Cu+2 + 2e− → Cu
• 3Cu2+ + 6e− → 3Cu
• Note: Do not allow credit for e without the (–) sign.
An operating voltaic cell has magnesium and silver electrodes. The cell and the ionic equation representing the reaction that occurs in the cell are shown below.
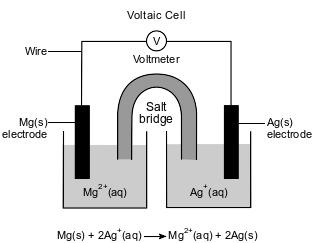
Write a balanced equation for the half-reaction that occurs at the magnesium electrode in this cell.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Mg(s) → Mg2+(aq) + 2e−
• Mg → Mg+2 + 2e−
• Note: Do not allow credit for e without the (−) sign.
A student sets up a voltaic cell using magnesium and zinc electrodes. The porous barrier in the cell has the same purpose as a salt bridge. The diagram and the ionic equation below represent this operating cell.
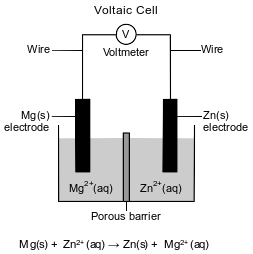
Write a balanced half-reaction equation for the oxidation that occurs in this operating cell.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Mg(s) → Mg2+(aq) + 2e−
• Mg → 2e− + Mg+2
A student constructs an electrochemical cell during a laboratory investigation. When the switch is closed, electrons flow through the external circuit. The diagram and ionic equation below represent this cell and the reaction that occurs.
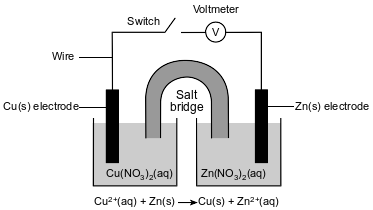
Write a balanced equation for the half-reaction that occurs in the Cu half-cell when the cell operates.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Cu2+(aq) + 2e− → Cu(s)
• 2e− + Cu+2 → Cu
Stamping an identification number into the steel frame of a bicycle compresses the crystal structure of the metal. If the number is filed off, there are scientific ways to reveal the number.
One method is to apply aqueous copper(II) chloride to the number area. The Cu2+ ions react with some iron atoms in the steel frame, producing copper atoms that show the pattern of the number. The ionic equation below represents this reaction.
Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s)
Another method is to apply hydrochloric acid to the number area. The acid reacts with the iron, producing bubbles of hydrogen gas. The bubbles form faster where the metal was compressed, so the number becomes visible. The equation below represents this reaction.
2HCl(aq) + Fe(s) → FeCl2(aq) + H2(g)
Write a balanced half-reaction equation for the reduction of the hydrogen ions to hydrogen gas.
Allow 1 credit. Acceptable responses include, but are not limited to:
• 2H+(aq) + 2e− → H2(g)
• 2H+ + 2e− → H2
• 2e− + 2H+1 → H2
A student develops the list shown below that includes laboratory equipment and materials for constructing a voltaic cell.

Complete and balance the half-reaction equation in your answer booklet for the oxidation of the Zn(s) that occurs in the voltaic cell.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Zn2+ + 2e−
• 2e− + Zn2+(aq)
• Zn+2 + 2e−
Early scientists defined oxidation as a chemical reaction in which oxygen combined with another element to produce an oxide of the element. An example of oxidation based on this definition is the combustion of methane. This reaction is represented by the balanced equation below.
Equation 1: CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
The definition of oxidation has since been expanded to include many reactions that do not involve oxygen. An example of oxidation based on this expanded definition is the reaction between magnesium ribbon and powdered sulfur when heated in a crucible. This reaction is represented by the balanced equation below.
Equation 2: Mg(s) + S(s) → MgS(s)
Write a balanced half-reaction equation for the oxidation that occurs in the reaction represented by equation 2.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Mg → Mg2+ + 2e−
• Mg − 2e− → Mg+2
A small digital clock can be powered by a battery made from two potatoes and some household materials. The “potato clock” battery consists of two cells connected in a way to produce enough electricity to allow the clock to operate. In each cell, zinc atoms react to form zinc ions. Hydrogen ions from phosphoric acid in the potatoes react to form hydrogen gas. The labeled diagram and balanced ionic equation below show the reaction, the materials, and connections necessary to make a “potato clock” battery.
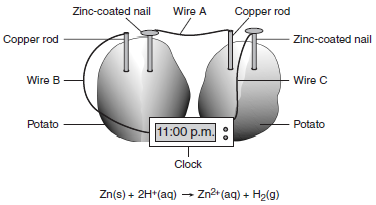
Write a balanced half-reaction equation for the oxidation that occurs in the “potato clock” battery.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Zn(s) → Zn2+(aq) + 2e−
• Zn − 2e− → Zn+2
Copper can be used for water pipes in homes. When the pipes corrode, copper atoms oxidize to form Cu2+ ions in the water.
A homeowner has a water quality report prepared for a sample of water taken from pipes in the home. According to the report, the 550.-gram sample contains 6.75 × 10−4 gram of dissolved Cu2+ ions.
Write a balanced half-reaction equation for the corrosion that forms the Cu2+ ions.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Cu → Cu2+ + 2e−
• Cu − 2e− → Cu+2
In a laboratory apparatus, a sample of lead(II) oxide reacts with hydrogen gas at high temperature. The products of this reaction are liquid lead and water vapor. As the reaction proceeds, water vapor and excess hydrogen gas leave the glass tube. The diagram and balanced equation below represent this reaction.
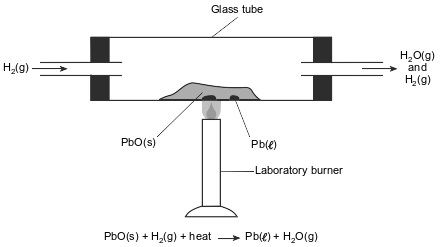
Write a balanced half-reaction equation for the reduction of the Pb2+ ions in this reaction.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Pb2+ + 2e− → Pb
