Topic: Heat And Temperature
Heat And Temperature
What is the amount of heat released by 1.00 gram of liquid water at 0°C when it changes to 1.00 gram of ice at 0°C?
(1) 4.18 J
(2) 273 J
(3) 334 J
(4) 2260 J
Given samples of water:
Sample 1: 100. grams of water at 10.°C Sample 2: 100. grams of water at 20.°C
Compared to sample 1, sample 2 contains
(1) molecules with a lower average kinetic energy
(2) molecules with a lower average velocity
(3) less heat energy
(4) more heat energy
The graph below represents the uniform heating of a substance from the solid to the gas phase.
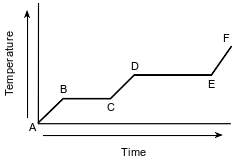
Which line segment of the graph represents boiling?
(1) AB—
(2) BC—
(3) CD—
(4) DE—
A beaker with water and the surrounding air are all at 24°C. After ice cubes are placed in the water, heat is transferred from
(1) the ice cubes to the air
(2) the beaker to the air
(3) the water to the ice cubes
(4) the water to the beaker
The average kinetic energy of the particles in a sample of matter is expressed as
(1) density
(2) volume
(3) pressure
(4) temperature
The temperature of a substance is a measure of the
(1) average kinetic energy of its particles
(2) average potential energy of its particles
(3) ionization energy of its particles
(4) activation energy of its particles
The average kinetic energy of water molecules decreases when
(1) H2O(ℓ) at 337 K changes to H2O(ℓ) at 300. K
(2) H2O(ℓ) at 373 K changes to H2O(g) at 373 K
(3) H2O(s) at 200. K changes to H2O(s) at 237 K
(4) H2O(s) at 273 K changes to H2O(ℓ) at 273 K
Which temperature represents the highest average kinetic energy of the particles in a sample of matter?
(1) 298 K
(2) 267 K
(3) 27°C
(4) 12°C
Water, H2O, and hexane, C6H14, are commonly used as laboratory solvents because they have different physical properties and are able to dissolve different types of solutes. Some physical properties of water and hexane are listed on the table below.
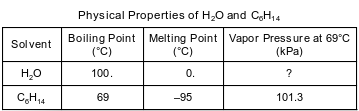
Compare the thermal energy of a 10.-gram sample of water at 25°C to the thermal energy of a 1000.-gram sample of water at 25°C.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The thermal energy is greater for the 1000 g sample of water.
• The smaller sample has less thermal energy.
Thermal energy is absorbed as chemical reactions occur during the process of baking muffins. The batter for muffins often contains baking soda, NaHCO3(s), which decomposes as the muffins are baked in an oven at 200.°C. The balanced equation below represents this reaction, which releases CO2(g) and causes the muffins to rise as they bake. The H2O(ℓ) is released into the air of the oven as it becomes a vapor.
2NaHCO3(s) + heat → Na2CO3(s) + H2O(ℓ) + CO2(g)
Compare the potential energy of the liquid water molecules to the potential energy of the water vapor molecules.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The potential energy of the liquid water molecules is less than the potential energy of the water vapor molecules.
• There is greater PE in the H2O(g).
- A test tube contains a sample of solid stearic acid, an organic acid.
- Both the sample and the test tube have a temperature of 22.0°C.
- The stearic acid melts after the test tube is placed in a beaker with
320. grams of water at 98.0°C.
- The temperature of the liquid stearic acid and water in the beaker reaches 74.0°C.
State the direction of heat transfer between the test tube and the water when the test tube was placed in the water.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Heat is transferred from the water to the test tube.
• The test tube absorbs thermal energy from the water.
• Stearic acid gained heat from the water.
Carbon dioxide, CO2, changes from the solid phase to the gas phase at 1 atm and 194.5 K. In the solid phase, CO2 is often called dry ice. When dry ice sublimes in air at 298 K, the water vapor in the air can condense, forming a fog of small water droplets. This fog is often used for special effects at concerts and in movie-making.
State the direction of heat flow between the dry ice and the water vapor in the air.
Allow 1 credit. Acceptable responses include, but are not limited to:
• from water vapor to the dry ice
• from H2O(g) to CO2(s)
• from water to CO2
A sample of a substance is a liquid at 65°C. The sample is heated uniformly to 125°C. The heating curve for the sample at standard pressure is shown below.

Determine the boiling point of the sample at standard pressure.
Allow 1 credit for any value from 94°C to 96°C, inclusive.
A student made a copper bracelet by hammering a small copper bar into the desired shape. The bracelet has a mass of 30.1 grams and was at a temperature of 21°C in the classroom. After the student wore the bracelet, the bracelet reached a temperature of 33°C. Later, the student removed the bracelet and placed it on a desk at home, where it cooled from 33°C to 19°C. The specific heat capacity of copper is 0.385 J/g•K.
Explain, in terms of heat flow, the change in the temperature of the bracelet when the student wore the bracelet.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The bracelet temperature increased because heat flowed from the body to the copper.
• Energy is tranferred from the student to the bracelet.
• Heat is absorbed by the bracelet.
A few pieces of dry ice, CO2(s), at −78°C are placed in a flask that contains air at 21°C. The flask is sealed by placing an uninflated balloon over the mouth of the flask. As the balloon inflates, the dry ice disappears and no liquid is observed in the flask.
State the direction of heat flow that occurs between the dry ice and the air in the flask.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Heat flows from the air in the flask to the dry ice.
• air to CO2
• to dry ice
• from air
