Topic: Heating Curves Calculation Of Heat
Heating Curves Calculation Of Heat
What is the amount of heat absorbed when the temperature of 75 grams of water increases from 20.°C to 35°C?
(1) 1100 J
(2) 4700 J
(3) 6300 J
(4) 11 000 J
Which numerical setup can be used to calculate the heat energy required to completely melt 100. grams of H2O(s) at 0°C?
(1) (100. g)(334 J/g)
(2) (100. g)(2260 J/g)
(3) (100. g)(4.18 J/g(cid:129)K)(0°C)
(4) (100. g)(4.18 J/g(cid:129)K)(273 K)
What is the amount of heat, in joules, required to increase the temperature of a 49.5-gram sample of water from 22°C to 66°C?
(1) 2.2 × 103 J
(2) 4.6 × 103 J
(3) 9.1 × 103 J
(4) 1.4 × 104 J
The cooling curve below represents the uniform cooling of a substance, starting at a temperature above its boiling point.

During which time interval does the substance exist as both a liquid and a solid?
(1) min 2 to min 4
(2) min 4 to min 5
(3) min 5 to min 7
(4) min 7 to min 9
As a 15.1-gram sample of a metal absorbs 48.75 J of heat, its temperature increases 25.0 K. What is the specific heat capacity of the metal?
(1) 0.129 J/g•K
(2) 1.95 J/g•K
(3) 3.23 J/g•K
(4) 7.74 J/g•K
What is the amount of heat required to completely melt a 200.-gram sample of H2O(s) at STP?
(1) 334 J
(2) 836 J
(3) 66 800 J
(4) 452 000 J
How many joules of heat are absorbed to raise the temperature of 435 grams of water at 1 atm from 25°C to its boiling point, 100.°C?
(1) 4.5 × 104 J
(2) 1.4 × 105 J
(3) 2.5 × 107 J
(4) 7.4 × 107 J
Water, H2O, and hexane, C6H14, are commonly used as laboratory solvents because they have different physical properties and are able to dissolve different types of solutes. Some physical properties of water and hexane are listed on the table below.
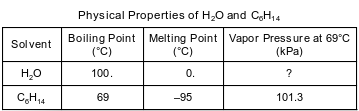
State what happens to the potential energy of the molecules in a solid sample of hexane at –95°C as heat is added until the hexane is completely melted.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The potential energy increases.
• P .E. goes up.
A 100.-gram sample of liquid water is heated from 20.0°C to 50.0°C. Enough KClO3(s) is dissolved in the sample of water at 50.0°C to form a saturated solution.
Using the information on Table B, determine the amount of heat absorbed by the water when the water is heated from 20.0°C to 50.0°C.
Allow 1 credit for 12 500 J or any value from 12 500 J to 13 000 J, inclusive.
The melting points and boiling points of fi ve substances at standard pressure are listed on the table below.
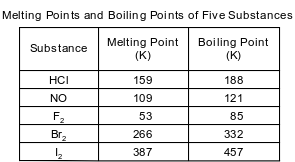
State what happens to the potential energy of a sample of NO(ℓ) at 121 K as it changes to NO(g) at constant temperature and standard pressure.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The potential energy of NO increases.
• The NO(ℓ) gains PE as it becomes NO(g).
Starting as a solid, a sample of a molecular substance is heated, until the entire sample of the substance is a gas. The graph below represents the relationship between the temperature of the sample and the elapsed time.
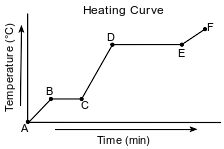
On the graph below, mark an X on the axis labeled “Temperature (°C)” to indicate the boiling point of the substance.
Allow 1 credit for an X marked on the axis labeled “Temperature (°C)” in line with interval
• DE.
• Example of a 1-credit response
• 
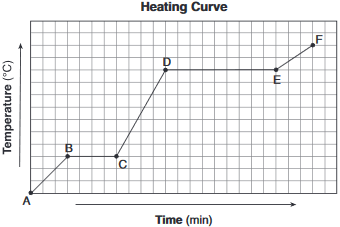
A sample of a molecular substance starting as a gas at 206°C and 1 atm is allowed to cool for 16 minutes. This process is represented by the cooling curve below.
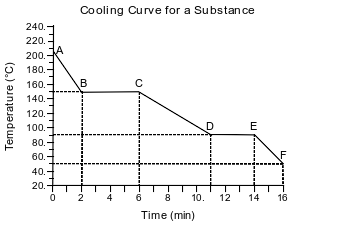
Describe what happens to the potential energy and the average kinetic energy of the molecules in the sample during interval DE.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Potential energy: decreases. Average kinetic energy: no change
• Potential energy: There is a decrease.. Average kinetic energy: It remains the same.
Show a numerical setup for calculating the quantity of heat in joules required to completely vaporize 102.3 grams of H2O(ℓ) at 100.°C and 1.0 atm.
Allow 1 credit. Acceptable responses include, but are not limited to:
• q = (102.3 g)(2260 J/g)
Fruit growers in Florida protect oranges when the temperature is near freezing by spraying water on them. It is the freezing of the water that protects the oranges from frost damage. When H2O(ℓ) at 0°C changes to H2O(s) at 0°C, heat energy is released. This energy helps to prevent the temperature inside the orange from dropping below freezing, which could damage the fruit. After harvesting, oranges can be exposed to ethene gas, C2H4, to improve their color.
Determine the quantity of heat released when 2.00 grams of H2O(ℓ) freezes at 0°C.
Allow 1 credit for 668 J or –668 J.
- A test tube contains a sample of solid stearic acid, an organic acid.
- Both the sample and the test tube have a temperature of 22.0°C.
- The stearic acid melts after the test tube is placed in a beaker with
320. grams of water at 98.0°C.
- The temperature of the liquid stearic acid and water in the beaker reaches 74.0°C.
Show a numerical setup for calculating the amount of thermal energy change for the water in the beaker.
Allow 1 credit. Acceptable responses include, but are not limited to:
• (320. g)(4.18 J/g•K)(98.0°C − 74.0°C)
• (320. g)(4.18 J/g•K)(347 K − 371 K)
• (320)(4.18)(24)
