Topic: Hydrocarbons
Hydrocarbons
Given the formula of a compound:

What is the molecular formula for this compound?
(1) CH
(2) CH2
(3) CH3
(4) C3H6
The only two elements in alkenes and alkynes are
(1) carbon and nitrogen
(2) carbon and hydrogen
(3) oxygen and nitrogen
(4) oxygen and hydrogen
Which compound is saturated?
(1) butane
(2) ethene
(3) heptene
(4) pentyne
Which hydrocarbon is saturated?
(1) C2H2
(2) C3H4
(3) C4H6
(4) C4H10
Which formula represents an unsaturated hydrocarbon?
(1) C2H4
(2) C3H8
(3) C4H10
(4) C5H12
Which formula represents a hydrocarbon?
(1) CH3I
(2) CH3NH2
(3) CH3CH3
(4) CH3OH
Hydrocarbons are composed of the elements
(1) carbon and hydrogen, only
(2) carbon and oxygen, only
(3) carbon, hydrogen, and oxygen
(4) carbon, nitrogen, and oxygen
A metal worker uses a cutting torch that operates by reacting acetylene gas, C2H2(g), with oxygen gas, O2(g), as shown in the unbalanced equation below.
C2H2(g) + O2(g) → CO2(g) + H2O(g) + heat
Explain, in terms of bonding, why the hydrocarbon gas used in the cutting torch is classified as an alkyne.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Each molecule has a triple carbon-to-carbon bond, C=−C.
• The two C atoms share 6 electrons.
• Each molecule has a triple bond.
• Alkynes have a C=−C.
The solvent 2-chloropropane can be made when chemists react propene with hydrogen chloride, as shown in the equation below.
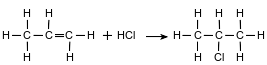
Write the general formula for the homologous series to which propene belongs.
Allow 1 credit for CnH2n.
Crude oil, primarily a mixture of hydrocarbons, is separated into useful components in a fractionating tower. At the bottom of the tower, the crude oil is heated to about 400°C. The gases formed rise and cool. Most of the gases condense and are collected as liquid fractions. The table below shows the temperature ranges for collecting various hydrocarbon fractions.
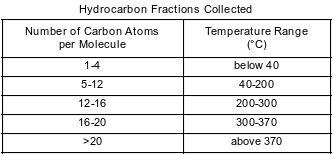
Determine the number of carbon atoms in one molecule of an alkane that has 22 hydrogen atoms in the molecule.
Allow 1 credit for 10 or ten.
The equation below represents an industrial preparation of diethyl ether.
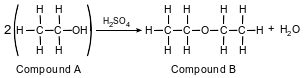
Explain, in terms of elements, why compound B is not a hydrocarbon.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Hydrocarbons contain only carbon and hydrogen, but compound B also contains oxygen.
• Compound B contains carbon, hydrogen, and a different element.
• This compound includes oxygen.
Natural gas and coal are two fuels burned to produce energy. Natural gas consists of approximately 80% methane, 10% ethane, 4% propane, 2% butane, and other components.
The burning of coal usually produces sulfur dioxide, SO2(g), and sulfur trioxide, SO3(g), which are major air pollutants. Both SO2(g) and SO3(g) react with water in the air to form acids.
Draw a structural formula for the hydrocarbon that is approximately 2% of natural gas.
Allow 1 credit.
• Examples of 1-credit responses:
• 
There are several isomers of C6H14. The formulas and boiling points for two of these isomers are given in the table below.

Identify the homologous series to which these isomers belong.
Allow 1 credit. Acceptable responses include, but are not limited to:
• alkanes
• CnH2n+2
Ethyl ethanoate is used as a solvent for varnishes and in the manufacture of artifi cial leather. The formula below represents a molecule of ethyl ethanoate.
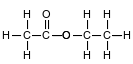
Determine the number of electrons shared in the bond between a hydrogen atom and a carbon atom in the molecule.
Allow 1 credit for 2 or two or 1 pair.
A thiol is very similar to an alcohol, but a thiol has a sulfur atom instead of an oxygen atom in the functional group. The equation below represents a reaction of methanethiol and iodine, producing dimethyl disulfide and hydrogen iodide.
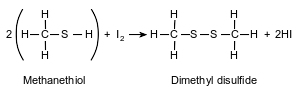
State the number of electrons shared between the sulfur atoms in the dimethyl disulfide.
Allow 1 credit for 2 or two or 1 pair.
