Topic: Lechateliers Principle
Lechateliers Principle
Given the equation representing a solution equilibrium:
![]()
What occurs when Na2SO4(s) is added to this system, increasing the concentration of SO42−(aq)?
(1) The equilibrium shifts to the left, and the concentration of Ba2+(aq) decreases.
(2) The equilibrium shifts to the left, and the concentration of Ba2+(aq) increases.
(3) The equilibrium shifts to the right, and the concentration of Ba2+(aq) decreases.
(4) The equilibrium shifts to the right, and the concentration of Ba2+(aq) increases.
Given the equation representing a system at equilibrium in a sealed, rigid container:
2HI(g) ⇄ H2(g) + I2(g) + energy
Increasing the temperature of the system causes the concentration of
(1) HI to increase
(2) H2 to increase
(3) HI to remain constant
(4) H2 to remain constant
Given the equation representing a system at equilibrium:
PCl5(g) + energy ⇌ PCl3(g) + Cl2(g)
Which change will cause the equilibrium to shift to the right?
(1) adding a catalyst
(2) adding more PCl3(g)
(3) increasing the pressure
(4) increasing the temperature
Which term identifies a factor that will shift a chemical equilibrium?
(1) atomic radius
(2) catalyst
(3) decay mode
(4) temperature
For a reaction system at equilibrium, LeChatelier’s principle can be used to predict the
(1) activation energy for the system
(2) type of bonds in the reactants
(3) effect of a stress on the system
(4) polarity of the product molecules
Given the equation representing a chemical reaction at equilibrium in a sealed, rigid container:
H2(g) + I2(g) + energy ⇌ 2HI(g)
When the concentration of H2(g) is increased by adding more hydrogen gas to the container at constant temperature, the equilibrium shifts
(1) to the right, and the concentration of HI(g) decreases
(2) to the right, and the concentration of HI(g) increases
(3) to the left, and the concentration of HI(g) decreases
(4) to the left, and the concentration of HI(g) increases
Given the equation representing a system at equilibrium:
![]()
Which change causes the equilibrium to shift?
(1) increasing pressure
(2) increasing temperature
(3) adding a noble gas
(4) adding a catalyst
Given the equation for a system at equilibrium: N2(g) + 3H2(g) ⇌ 2NH3(g) + energy
If only the concentration of N2(g) is increased, the concentration of
(1) NH3(g) increases
(2) NH3(g) remains the same
(3) H2(g) increases
(4) H2(g) remains the same
Nitrogen dioxide, NO2, is a dark brown gas that is used to make nitric acid and to bleach flour. Nitrogen dioxide has a boiling point of 294 K at 101.3 kPa. In a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, N2O4. This equilibrium is represented by the equation below.
2NO2(g) ⇌ N2O4(g) + 58 kJ
State one stress, other than adding or removing NO2(g) or N2O4(g), that would increase the amount of the dark brown gas.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Increase the temperature.
• Add heat.
• Decrease the pressure.
• Increase the volume.
An equilibrium system in a sealed, rigid container is represented by the equation below.
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
State the effect on the concentrations of H2O(g) and CO2(g) when more H2(g) is added to the system.
Allow 1 credit. Acceptable responses include, but are not limited to:
• H2O(g): increases/higher
• CO2(g): decreases/lower
Stamping an identification number into the steel frame of a bicycle compresses the crystal structure of the metal. If the number is filed off, there are scientific ways to reveal the number.
One method is to apply aqueous copper(II) chloride to the number area. The Cu2+ ions react with some iron atoms in the steel frame, producing copper atoms that show the pattern of the number. The ionic equation below represents this reaction.
Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s)
Another method is to apply hydrochloric acid to the number area. The acid reacts with the iron, producing bubbles of hydrogen gas. The bubbles form faster where the metal was compressed, so the number becomes visible. The equation below represents this reaction.
2HCl(aq) + Fe(s) → FeCl2(aq) + H2(g)
Describe one change in the HCl(aq) that will increase the rate at which hydrogen bubbles are produced when the acid is applied to the steel frame.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Increase the concentration of the HCl(aq).
• Increase the temperature.
The equation below represents an equilibrium system of SO2(g), O2(g), and SO3(g). The reaction can be catalyzed by vanadium or platinum.
2SO2(g) + O2(g) ⇌ 2SO3(g) + energy
State how the equilibrium shifts when SO3(g) is removed from the system.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The equilibrium will shift to favor the formation of SO3.
• The rate of the forward reaction is greater than the rate of the reverse reaction.
• The equilibrium will shift to favor the forward reaction.
• The equilibrium will shift to the right.
• The concentrations of the reactants will decrease.
A student makes an aqueous solution of lactic acid. A formula for one form of lactic acid is shown below.
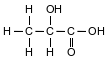
The solution is placed in a sealed flask to be used in a laboratory investigation. The equation below represents the lactic acid equilibrium system in the flask.
![]()
Explain, in terms of LeChatelier’s principle, why increasing the concentration of H+(aq) increases the concentration of lactic acid.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The stress of adding H+ ions shifts the equilibrium to the left, producing more lactic acid.
• Increasing the concentration of H+(aq) favors the reverse reaction.
• More H+ ions collide with lactate ions, shifting the equilibrium left.
Common household bleach is an aqueous solution containing hypochlorite ions. A closed container of bleach is an equilibrium system represented by the equation below.
Cl2(g) + 2OH−(aq) ⇌ ClO−(aq) + Cl−(aq) + H2O(ℓ)
State the effect on the concentration of the ClO− ion when there is a decrease in the concentration of the OH− ion.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The concentration of the ClO− ion decreases.
• [ClO−] decreases.
• lower ClO− concentration
• less ClO−
Several steps are involved in the industrial production of sulfuric acid. One step involves the oxidation of sulfur dioxide gas to form sulfur trioxide gas. A catalyst is used to increase the rate of production of sulfur trioxide gas. In a rigid cylinder with a movable piston, this reaction reaches equilibrium, as represented by the equation below.
2SO2(g) + O2(g) ⇌ 2SO3(g) + 392 kJ
State, in terms of the concentration of SO3(g), what occurs when more O2(g) is added to the reaction at equilibrium.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The concentration of SO3(g) increases.
