Topic: Mode And Rate Of Decay
Mode And Rate Of Decay
Based on Table N, uranium-238 and uranium-235 have different
(1) decay modes
(2) half-lives
(3) numbers of protons
(4) numbers of electrons
What is the mass of an original 5.60-gram sample of iron-53 that remains unchanged after 25.53 minutes?
(1) 0.35 g
(2) 0.70 g
(3) 1.40 g
(4) 2.80 g
Which radioisotope has the fastest rate of decay?
(1) 14C
(2) 37Ca
(3) 53Fe
(4) 42K
Which phrase describes the decay modes and the half-lives of K-37 and K-42?
(1) the same decay mode but different half-lives
(2) the same decay mode and the same half-life
(3) different decay modes and different half-lives
(4) different decay modes but the same half-life
A radioactive isotope has a half-life of 2.5 years. Which fraction of the original mass remains unchanged after 10. years?
(1) 1/2
(2) 1/4
(3) 1/8
(4) 1/16
Compared to the half-life and decay mode of the nuclide 90Sr, the nuclide 226Ra has
(1) a longer half-life and the same decay mode
(2) a longer half-life and a different decay mode
(3) a shorter half-life and the same decay mode
(4) a shorter half-life and a different decay mode
What fraction of a Sr-90 sample remains unchanged after 87.3 years?
(1) 1/2
(2) 1/3
(3) 1/4
(4) 1/8
Phosphorus-30 and phosphorus-32 are radioisotopes. Phosphorus-30 decays by positron emission.
Based on Table N, determine the time required for an original 100.-milligram sample of P-32 to decay until only 25 milligrams of the sample remain unchanged.
Allow 1 credit for 28.56 d. Significant figures do not need to be shown.
Radioactive emissions can be detected by a Geiger counter. When radioactive emissions enter the Geiger counter probe, which contains a noble gas such as argon or helium, some of the atoms are ionized. The ionized gas allows for a brief electric current. The current causes the speaker to make a clicking sound. To make sure that the Geiger counter is measuring radiation properly, the device is tested using the radioisotope Cs-137.
To detect gamma radiation, an aluminum shield can be placed over the probe window, to keep alpha and beta radiation from entering the probe. A diagram that represents the Geiger counter is shown below.
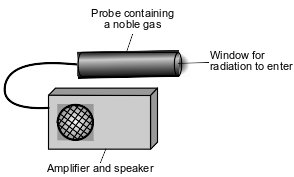
Determine the time required for a sample of cesium-137 to decay until only 1/8 of the original sample remains unchanged.
Allow 1 credit for 90.6 y. Significant figures do not need to be shown.
Cobalt-60 is an artifi cial isotope of Co-59. The incomplete equation for the decay of cobalt-60, including beta and gamma emissions, is shown below.
![]()
Based on Table N, determine the total time required for an 80.00-gram sample of cobalt-60 to decay until only 10.00 grams of the sample remain unchanged.
Allow 1 credit for 15.813 y. Significant figures do not need to be shown.
In the past, some paints that glowed in the dark contained zinc sulfide and salts of Ra-226. As the radioisotope Ra-226 decayed, the energy released caused the zinc sulfide in these paints to emit light. The half-lives for Ra-226 and two other radioisotopes used in these paints are listed on the table below.
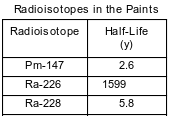
What fraction of an original Ra-228 sample remains unchanged after 17.4 years?
Allow 1 credit. Acceptable responses include, but are not limited to:
• 1/8
• 0.125
• 12.5%
Some isotopes of neon are Ne-19, Ne-20, Ne-21, Ne-22, and Ne-24. The neon-24 decays by beta emission. The atomic mass and natural abundance for the naturally occurring isotopes of neon are shown in the table below.
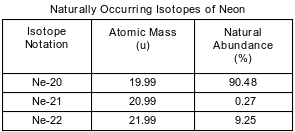
Identify the decay mode of Ne-19.
Allow 1 credit. Acceptable responses include, but are not limited to:
• positron decay
• β+
• 0+1e
• 0+1β
• positron
When uranium-235 nuclei are bombarded with neutrons, many different combinations of smaller nuclei can be produced. The production of neodymium-150 and germanium-81 in one of these reactions is represented by the equation below.
![]()
Germanium-81 and uranium-235 have different decay modes. Ge-81 emits beta particles and has a half-life of 7.6 seconds.
Determine the time required for a 16.00-gram sample of Ge-81 to decay until only 1.00 gram of the sample remains unchanged.
Allow 1 credit for 30.4 s. Significant figures do not need to be shown.
When a cobalt-59 atom is bombarded by a subatomic particle, a radioactive cobalt-60 atom is produced. After 21.084 years, 1.20 grams of an original sample of cobalt-60 produced remains unchanged.
Determine the mass of the original sample of cobalt-60 produced.
Allow 1 credit for 19.20 g. Significant figures do not need to be shown.
The diagram below shows the first three steps in the uranium-238 radioactive decay series.
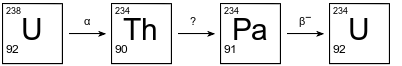
The decay mode for the first and third steps is shown above the arrows. The decay mode for the second step is not shown in the diagram. Thorium-234 has a half-life of 24.10 days.
Determine the total time that must elapse until only 1/16 of an original sample of Th-234 remains unchanged.
Allow 1 credit for 96.40 d. Significant figures do not need to be shown.
