Topic: Nucleus
Nucleus
Which particles are found in the nucleus of an argon atom?
(1) protons and electrons
(2) positrons and neutrons
(3) protons and neutrons
(4) positrons and electrons
The part of an atom that has an overall positive charge is called
(1) an electron
(2) the nucleus
(3) the first shell
(4) the valence shell
Which statement describes the charge and location of an electron in an atom?
(1) An electron has a positive charge and is located outside the nucleus.
(2) An electron has a positive charge and is located in the nucleus.
(3) An electron has a negative charge and is located outside the nucleus.
(4) An electron has a negative charge and is located in the nucleus.
Which phrase describes an Al atom?
(1) a negatively charged nucleus, surrounded by negatively charged electrons
(2) a negatively charged nucleus, surrounded by positively charged electrons
(3) a positively charged nucleus, surrounded by negatively charged electrons
(4) a positively charged nucleus, surrounded by positively charged electrons
Which statement describes the structure of an atom?
(1) The nucleus contains positively charged electrons.
(2) The nucleus contains negatively charged protons.
(3) The nucleus has a positive charge and is surrounded by negatively charged electrons.
(4) The nucleus has a negative charge and is surrounded by positively charged electrons.
What is the charge of the nucleus of a copper atom?
(1) +1
(2) +2
(3) +29
(4) +64
Which statement describes the location of two types of subatomic particles in a helium atom?
(1) Protons and neutrons are located in the nucleus.
(2) Protons and neutrons are located outside the nucleus.
(3) Protons and electrons are located in the nucleus.
(4) Protons and electrons are located outside the nucleus.
Which subatomic particles are found in the nucleus of an atom of beryllium?
(1) electrons and protons
(2) electrons and positrons
(3) neutrons and protons
(4) neutrons and electrons
Which phrase describes the charge and mass of a neutron?
(1) a charge of +1 and no mass
(2) a charge of +1 and an approximate mass of 1 u
(3) no charge and no mass
(4) no charge and an approximate mass of 1 u
According to the modern model of the atom, the nucleus of an atom is surrounded by one or more
(1) electrons
(2) neutrons
(3) positrons
(4) protons
Which phrase describes an atom?
(1) a negatively charged nucleus surrounded by positively charged protons
(2) a negatively charged nucleus surrounded by positively charged electrons
(3) a positively charged nucleus surrounded by negatively charged protons
(4) a positively charged nucleus surrounded by negatively charged electrons
What is the charge of the nucleus of an oxygen atom?
(1) 0
(2) −2
(3) +8
(4) +16
The diagrams below represent four different atomic nuclei.
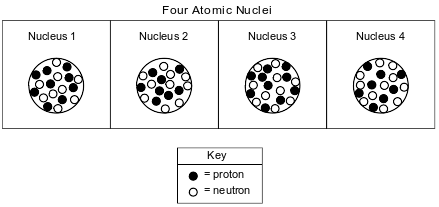
Identify the nucleus above that is found in an atom that has a stable valence electron configuration.
Allow 1 credit. Acceptable responses include, but are not limited to:
• nucleus 3
Elements with an atomic number greater than 92 can be artificially produced in nuclear reactions by bombarding a naturally occurring nuclide with a different nuclide. One of these elements is roentgenium, Rg. The equation below represents a nuclear reaction that produces Rg-272.
![]()
State the location and the total charge of the protons in a Ni-64 atom.
Allow 1 credit for a correct combination of the location and the total charge of the protons.
• Acceptable responses include, but are not limited to:
• Location of protons:
• in the nucleus
• the small, dense center of an atom
• center of an atom
• Total charge of protons:
• +28
• 28+
• 28
The element boron, a trace element in Earth’s crust, is found in foods produced from plants. Boron has only two naturally occurring stable isotopes, boron-10 and boron-11.
State, in terms of subatomic particles, one difference between the nucleus of a carbon-11 atom and the nucleus of a boron-11 atom.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The carbon-11 nucleus has one more proton than the nucleus of boron-11.
• A B-11 atom has a different number of neutrons than a C-11 atom.
