Topic: Organic Compounds
Organic Compounds
Given the formula for a compound:
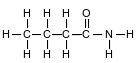
What is a chemical name for the compound?
(1) 1-butanamine
(2) 1-butanol
(3) butanamide
(4) butanoic acid
Which organic prefix is matched with the number of carbon atoms that it represents?
(1) hept-, 7
(2) non-, 8
(3) pent-, 3
(4) prop-, 4
Given the formula of a compound:
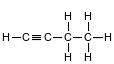
This compound is classifi ed as an
(1) aldehyde
(2) alkene
(3) alkyne
(4) alcohol
Given the formula representing a molecule:
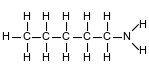
A chemical name for this compound is
(1) pentanone
(2) 1-pentanol
(3) 1-pentanamine
(4) pentanamide
What is the general formula for the homologous series that includes ethene?
(1) CnH2n
(2) CnH2n−6
(3) CnH2n−2
(4) CnH2n+2
The atoms of which element bond to one another in chains, rings, and networks?
(1) barium
(2) carbon
(3) iodine
(4) mercury
Given the formula for a compound:
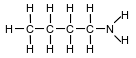
What is a chemical name for this compound?
(1) 1-butanamide
(2) 4-butanamide
(3) 1-butanamine
(4) 4-butanamine
What is the chemical name for the compound CH3CH2CH2CH3?
(1) butane
(2) butene
(3) decane
(4) decene
Which formula can represent an alkyne?
(1) C2H4
(2) C2H6
(3) C3H4
(4) C3H6
A molecule of any organic compound has at least one
(1) ionic bond
(2) double bond
(3) oxygen atom
(4) carbon atom
The electrical conductivity of three aqueous solutions was tested at room temperature. A 0.1 M HCl(aq) solution conducted, but a 6.0 M HCl(aq) solution was a better conductor. A 0.1 M C6H12O6(aq) solution was also tested. During this laboratory activity, appropriate safety equipment was used and safety procedures were followed.
Identify the element in C6H12O6 that allows it to be classified as an organic compound.
Allow 1 credit for C or carbon.
The solvent 2-chloropropane can be made when chemists react propene with hydrogen chloride, as shown in the equation below.
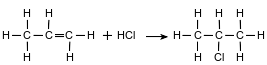
Identify the element in propene that is in all organic compounds.
Allow 1 credit for C or carbon.
Ethyl ethanoate is used as a solvent for varnishes and in the manufacture of artifi cial leather. The formula below represents a molecule of ethyl ethanoate.
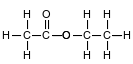
Identify the element in ethyl ethanoate that makes it an organic compound.
Allow 1 credit for C or carbon.
The equation below represents an industrial preparation of diethyl ether.
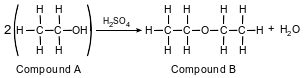
Identify the element in compound B that makes it an organic compound.
Allow 1 credit for C or carbon.
Diethyl ether is used as a laboratory and industrial solvent. The boiling point of diethyl ether at standard pressure is 34.6°C. The equation below represents a reaction that produces diethyl ether.
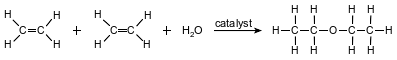
Identify the element in diethyl ether that allows it to be classified as an organic compound.
Allow 1 credit for carbon or C.
