Topic: Oxidation Reduction
Oxidation Reduction
Which particles are transferred during a redox reaction?
(1) atoms
(2) electrons
(3) neutrons
(4) positrons
Given the balanced ionic equation representing a reaction:
Zn(s) + Co2+(aq) → Zn2+(aq) + Co(s)
Which statement describes the electrons involved in this reaction?
(1) Each Zn atom loses 2 electrons, and each Co2+ ion gains 2 electrons.
(2) Each Zn atom loses 2 electrons, and each Co2+ ion loses 2 electrons.
(3) Each Zn atom gains 2 electrons, and each Co2+ ion loses 2 electrons.
(4) Each Zn atom gains 2 electrons, and each Co2+ ion gains 2 electrons.
Given the balanced ionic equation representing a reaction:
Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(s)
During this reaction, electrons are transferred from
(1) Cu(s) to Ag+(aq)
(2) Cu2+(aq) to Ag(s)
(3) Ag(s) to Cu2+(aq)
(4) Ag+(aq) to Cu(s)
Which process involves the transfer of electrons?
(1) double replacement
(2) neutralization
(3) oxidation-reduction
(4) sublimation
Which ionic equation represents a spontaneous reaction that can occur in a voltaic cell?
(1) Cu(s) + Zn(s) → Cu2+(aq) + Zn2+(aq)
(2) Cu(s) + Zn2+(aq) → Cu2+(aq) + Zn(s)
(3) Cu2+(aq) + Zn(s) → Cu(s) + Zn2+(aq)
(4) Cu2+(aq) + Zn2+(aq) → Cu(s) + Zn(s)
Which type of reaction involves the transfer of electrons?
(1) alpha decay
(2) double replacement
(3) neutralization
(4) oxidation-reduction
Which balanced equation represents a redox reaction?
(1) Mg + Cl2 → MgCl2
(2) CaO + H2O → Ca(OH)2
(3) HNO3 + NaOH → NaNO3 + H2O
(4) NaCl + AgNO3 → AgCl + NaNO3
Given the balanced equation representing a reaction:
2Al(s) + 3Cu2+(aq) → 2Al3+(aq) + 3Cu(s)
Which particles are transferred in this reaction?
(1) electrons
(2) neutrons
(3) positrons
(4) protons
In which type of chemical reaction are electrons transferred?
(1) organic addition
(2) oxidation-reduction
(3) double replacement
(4) acid-base neutralization
Given the incomplete equation representing a reaction:
![]()
What is the formula of the missing product?
(1) O2−
(2) O2
(3) OH−
(4) OH
Stamping an identification number into the steel frame of a bicycle compresses the crystal structure of the metal. If the number is filed off, there are scientific ways to reveal the number.
One method is to apply aqueous copper(II) chloride to the number area. The Cu2+ ions react with some iron atoms in the steel frame, producing copper atoms that show the pattern of the number. The ionic equation below represents this reaction.
Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s)
Another method is to apply hydrochloric acid to the number area. The acid reacts with the iron, producing bubbles of hydrogen gas. The bubbles form faster where the metal was compressed, so the number becomes visible. The equation below represents this reaction.
2HCl(aq) + Fe(s) → FeCl2(aq) + H2(g)
Explain why the Fe atoms in the bicycle frame react with the Cu2+ ions.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Fe oxidizes in the presence of Cu2+ ions.
• Iron is a more active metal than copper.
• Cu2 ions act as an oxidizing agent.
• Fe is above Cu on Table J.
Fossil fuels produce air pollution and may eventually be depleted. Scientists are researching ways to use hydrogen as an alternate fuel.
A device called an artificial leaf was invented to produce hydrogen and oxygen using sunlight and water. The artifical leaf is an electrochemical cell. Equations 1 and 2 below represent the reactions taking place in the leaf. Equation 3 represents a reaction of hydrogen when used as fuel.
Equation 1: 2H2O + energy from sunlight → O2 + 4H+ + 4e−
Equation 2: 4H+ + 4e− → 2H2
Equation 3: 2H2(g) + O2(g) → 2H2O(g) + energy
State one benefit of using the artificial leaf to produce hydrogen.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The hydrogen could replace the use of fossil fuel.
• The use of hydrogen as a car fuel could reduce air pollution.
• The H2 fuel is renewable.
• Water is a nonpolluting product.
• The leaf uses renewable resources.
Early scientists defined oxidation as a chemical reaction in which oxygen combined with another element to produce an oxide of the element. An example of oxidation based on this definition is the combustion of methane. This reaction is represented by the balanced equation below.
Equation 1: CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
The definition of oxidation has since been expanded to include many reactions that do not involve oxygen. An example of oxidation based on this expanded definition is the reaction between magnesium ribbon and powdered sulfur when heated in a crucible. This reaction is represented by the balanced equation below.
Equation 2: Mg(s) + S(s) → MgS(s)
State why early scientists classified the reaction represented by equation 1 as oxidation.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Oxides are formed.
• A substance reacts with oxygen.
The diagram and ionic equation below represent an operating voltaic cell.
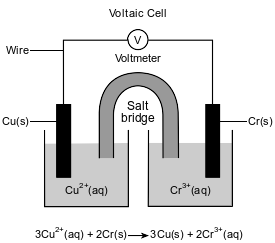
Write a balanced equation for the half-reaction that occurs in the copper half-cell when the cell operates.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Cu2+(aq) + 2e− → Cu(s)
• Cu+2 + 2e− → Cu
• 3Cu2+ + 6e− → 3Cu
• Note: Do not allow credit for e without the (–) sign.
An operating voltaic cell has magnesium and silver electrodes. The cell and the ionic equation representing the reaction that occurs in the cell are shown below.
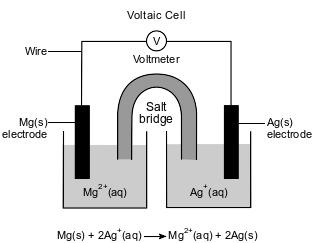
Write a balanced equation for the half-reaction that occurs at the magnesium electrode in this cell.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Mg(s) → Mg2+(aq) + 2e−
• Mg → Mg+2 + 2e−
• Note: Do not allow credit for e without the (−) sign.
