Topic: Separation Of The Components Of A Mixture
Separation Of The Components Of A Mixture
Distillation is a process used to separate a mixture of liquids based on different
(1) boiling points
(2) densities
(3) freezing points
(4) solubilities
Table sugar can be separated from a mixture of table sugar and sand at STP by adding
(1) sand, stirring, and distilling at 100.°C
(2) sand, stirring, and fi ltering
(3) water, stirring, and distilling at 100.°C
(4) water, stirring, and fi ltering
A mixture consists of ethanol and water. Some properties of ethanol and water are given in the table below.
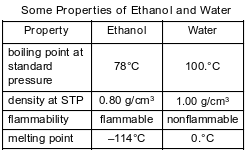
Which statement describes a property of ethanol after being separated from the mixture?
(1) Ethanol is nonflammable.
(2) Ethanol has a melting point of 0.°C.
(3) Ethanol has a density of 0.80 g/cm3 at STP.
(4) Ethanol has a boiling point of 89°C at standard pressure.
In a laboratory investigation, a student separates colored compounds obtained from a mixture of crushed spinach leaves and water by using paper chromatography. The colored compounds separate because of differences in
(1) molecular polarity
(2) malleability
(3) boiling point
(4) electrical conductivity
Paper chromatography can separate the components of a mixture of colored dyes because the components have differences in
(1) decay mode
(2) thermal conductivity
(3) ionization energy
(4) molecular polarity
At STP, two 5.0-gram solid samples of different ionic compounds have the same density. These solid samples could be differentiated by their
(1) mass
(2) volume
(3) temperature
(4) solubility in water
Given the formulas representing two compounds at standard pressure:
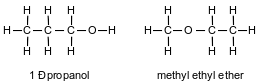
The compounds can be differentiated by their
(1) boiling points
(2) gram-formula masses
(3) numbers of hydrogen atoms
(4) percent compositions by mass of carbon
Differences in which property allow the separation of a sample of sand and seawater by filtration?
(1) concentration of ions
(2) volume of sample
(3) mass of sample
(4) particle size
The graph below shows the volume and the mass of four different substances at STP.
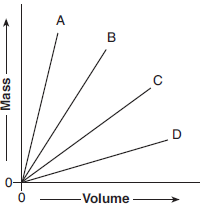
Which of the four substances has the lowest density?
(1) A
(2) B
(3) C
(4) D
During a laboratory activity, appropriate safety equipment is used and safety procedures are followed. A student separates a sample of rock salt that has two components; NaCl(s) and small insoluble rock particles. First, the student thoroughly stirs the sample of rock salt into a sample of water in a fl ask. The mixture in the fl ask is fi ltered using the lab apparatus shown below.
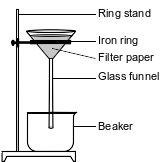
The water is evaporated from the beaker. The fi lter paper and its contents are dried. The data collected by the student are shown in the table below.
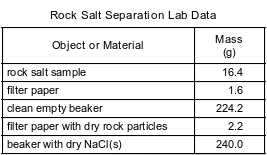
Explain, in terms of particle size, why the rock particles are trapped by the fi lter paper.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The particles of the rock are much larger than the openings in the filter paper.
• The rock particles are too big to pass through the paper.
A hydrate is a compound that has water molecules within its crystal structure. Magnesium sulfate heptahydrate, MgSO4•7H2O, is a hydrated form of magnesium sulfate. The hydrated compound has 7 moles of H2O for each mole of MgSO4. When 5.06 grams of MgSO4•7H2O are heated to at least 300.°C in a crucible by using a laboratory burner, the water molecules are released. The sample was heated repeatedly, until the remaining MgSO4 had a constant mass of 2.47 grams. During this laboratory activity, appropriate safety equipment was used and safety procedures were followed.
Explain why the sample in the crucible was heated repeatedly until the sample had a constant mass.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Repeated heatings ensure that all of the water in the sample has been removed.
• It is necessary to drive out all of the water from the hydrate.
• to make sure all the H2O is gone
Crude oil, primarily a mixture of hydrocarbons, is separated into useful components in a fractionating tower. At the bottom of the tower, the crude oil is heated to about 400°C. The gases formed rise and cool. Most of the gases condense and are collected as liquid fractions. The table below shows the temperature ranges for collecting various hydrocarbon fractions.
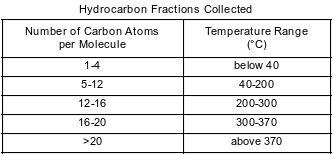
State the temperature range for the fraction collected that contains octane molecules.
Allow 1 credit for 40°C to 200°C. Significant figures do not need to be shown.
In a laboratory investigation, a student is given a sample that is a mixture of 3.0 grams of NaCl(s) and 4.0 grams of sand, which is mostly SiO2(s). The purpose of the investigation is to separate and recover the compounds in the sample. In the first step, the student places the sample in a 250-mL flask. Then, 50. grams of distilled water are added to the flask, and the contents are thoroughly stirred. The mixture in the flask is then filtered, using the equipment represented by the diagram below.
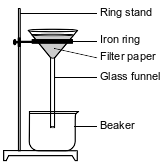
Describe a procedure to remove the water from the mixture that passes through the filter and collects in the beaker.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Allow the water to evaporate.
• Heat the mixture until all of the water vaporizes.
• Boil off the water.
Identify a laboratory process that can be used to separate a liquid mixture of methanol and water, based on the differences in their boiling points.
Allow 1 credit. Acceptable responses include, but are not limited to:
• distillation
• distilling
State the physical property that makes it possible to separate a solution by distillation.
Allow 1 credit. Acceptable responses include, but are not limited to:
• boiling point
• boiling temperature
