Topic: States Of Matter
States Of Matter
In which 1.0-gram sample are the particles arranged in a crystal structure?
(1) CaCl2(s)
(2) C2H6(g)
(3) CH3OH(ℓ)
(4) CaI2(aq)
One mole of liquid water and one mole of solid water have different
(1) masses
(2) properties
(3) empirical formulas
(4) gram-formula masses
Which sample of matter has a crystal structure?
(1) Hg(ℓ)
(2) H2O(ℓ)
(3) NaCl(s)
(4) CH4(g)
At 1 atm, equal masses of H2O(s), H2O(ℓ), and H2O(g) have
(1) the same density
(2) the same distance between molecules
(3) different volumes
(4) different percent compositions
Some physical properties of two samples of iodine-127 at two different temperatures are shown in the table below.
These two samples are two different
(1) mixtures
(2) substances
(3) phases of matter
(4) isotopes of iodine
A sample of CO2(s) and a sample of CO2(g) differ in their
(1) chemical compositions
(2) empirical formulas
(3) molecular structures
(4) physical properties
Which sample of matter sublimes at room temperature and standard pressure?
(1) Br2(ℓ)
(2) Cl2(g)
(3) CO2(s)
(4) SO2(aq)
Which sample of CO2 has a definite shape and a definite volume?
(1) CO2(aq)
(2) CO2(g)
(3) CO2(ℓ)
(4) CO2(s)
Which particle diagram represents a sample of oxygen gas at STP?
![]()
(1) 
(2) 
(3) 
(4) 
Which particle model diagram represents xenon at STP?

(1) 
(2) 
(3) 
(4) 
A metal worker uses a cutting torch that operates by reacting acetylene gas, C2H2(g), with oxygen gas, O2(g), as shown in the unbalanced equation below.
C2H2(g) + O2(g) → CO2(g) + H2O(g) + heat
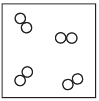
In your answer booklet, use the key to draw a particle model diagram to represent the phase of the O2(g). Your response must include at least six molecules.
Allow 1 credit for a diagram with at least six diatomic molecules drawn to represent the gas phase of
• the sample.
• Example of a 1-credit response:
• 
Starting as a solid, a sample of a molecular substance is heated, until the entire sample of the substance is a gas. The graph below represents the relationship between the temperature of the sample and the elapsed time.
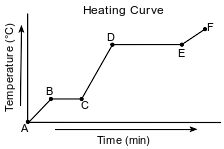
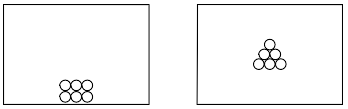
Using the key below, draw a particle diagram to represent the sample during interval AB. Your response must include at least six molecules.

Allow 1 credit for a diagram with at least six molecules drawn to represent the solid phase of
• the sample.
• Examples of 1-credit responses:
• 
In the late 1800s, Dmitri Mendeleev developed a periodic table of the elements known at that time. Based on the pattern in his periodic table, he was able to predict properties of some elements that had not yet been discovered. Information about two of these elements is shown in the table below.
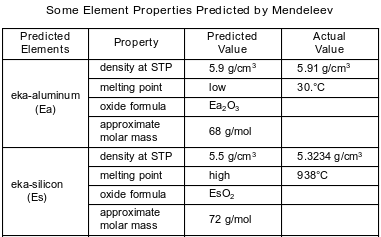
Identify the phase of Ea at 310. K.
Allow 1 credit for liquid or (ℓ).
The table below contains selected information about chlorine and two compounds containing chlorine. One piece of information is missing for each of the substances in the table.
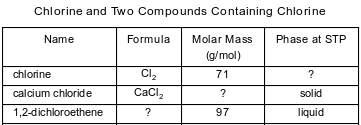
Identify the phase of the chlorine at STP.
Allow 1 credit. Acceptable responses include, but are not limited to:
• gas
• (g)
A technician recorded data for two properties of Period 3 elements. The data are shown in the table below.
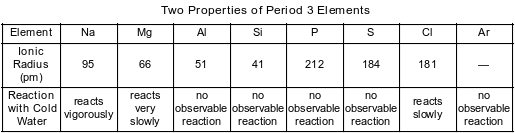
State the phase of chlorine at 281 K and 101.3 kPa.
Allow 1 credit for gas or (g).
