Topic: Chemical Bonding
Chemical Bonding
Which statement describes H2O(ℓ) and H2O2(ℓ)?
(1) Both are compounds that have the same properties.
(2) Both are compounds that have different properties.
(3) Both are mixtures that have the same properties.
(4) Both are mixtures that have different properties.
Carbon monoxide and carbon dioxide have
(1) the same chemical properties and the same physical properties
(2) the same chemical properties and different physical properties
(3) different chemical properties and the same physical properties
(4) different chemical properties and different physical properties
Chemical properties can be used to
(1) determine the temperature of a substance
(2) determine the density of a substance
(3) differentiate between two compounds
(4) differentiate between two neutrons
Compared to the physical and chemical proper- ties of the compound NO2, the compound N2O has
(1) different physical properties and different chemical properties
(2) different physical properties and the same chemical properties
(3) the same physical properties and different chemical properties
(4) the same physical properties and the same chemical properties
A sample of a substance has these characteristics: (cid:129) melting point of 984 K (cid:129) hard, brittle solid at room temperature (cid:129) poor conductor of heat and electricity as a solid (cid:129) good conductor of electricity as a liquid or in
an aqueous solution
This sample is classified as
(1) a metallic element
(2) a radioactive element
(3) a molecular compound
(4) an ionic compound
Which formulas represent one ionic compound and one molecular compound?
(1) N2 and SO2
(2) Cl2 and H2S
(3) BaCl2 and N2O4
(4) NaOH and BaSO4
Which terms identify two different categories of compounds?
(1) covalent and molecular
(2) covalent and empirical
(3) ionic and molecular
(4) ionic and empirical
Which two terms represent major categories of compounds?
(1) ionic and nuclear
(2) ionic and molecular
(3) empirical and nuclear
(4) empirical and molecular
The table below shows properties of two compounds at standard pressure.
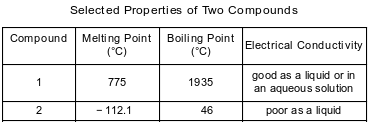
Which statement classifies the two compounds?
(1) Both compounds are ionic.
(2) Both compounds are molecular.
(3) Compound 1 is ionic, and compound 2 is molecular.
(4) Compound 1 is molecular, and compound 2 is ionic.
A solid sample of a compound and a liquid sample of the same compound are each tested for electrical conductivity. Which test conclusion indicates that the compound is ionic?
(1) Both the solid and the liquid are good conductors.
(2) Both the solid and the liquid are poor conductors.
(3) The solid is a good conductor, and the liquid is a poor conductor.
(4) The solid is a poor conductor, and the liquid is a good conductor.
A laboratory technician is given the table below and a sample of one of the three substances listed in the table. The technician makes an aqueous solution with a portion of the sample. When a conductivity tester is lowered into the solution, the lightbulb on the tester glows brightly. Another portion of the sample is placed in a heat-resistant container that is placed in an oven at 450.°C. The sample melts.
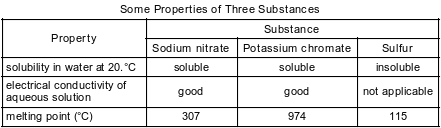
State evidence that makes it necessary to use more than one property to identify the substance given to the technician.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Sodium nitrate and potassium chromate are both soluble in water and are good conductors in solution. Therefore, the melting points are needed to identify the substance.
• Solubility alone cannot be used because two of the substances are soluble in water.
• Two of the substances melt below 450.ºC.
• Electrical conductivity is not sufficient to differentiate the two salts.
Fatty acids, a class of compounds found in living things, are organic acids with long hydrocarbon chains. Linoleic acid, an unsaturated fatty acid, is essential for human skin flexibility and smoothness. The formula below represents a molecule of linoleic acid.
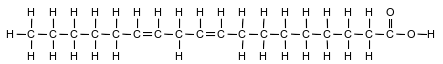
Identify the type of chemical bond between the oxygen atom and the hydrogen atom in the linoleic acid molecule.
Allow 1 credit. Acceptable responses include, but are not limited to:
• covalent
• polar covalent
• polar covalent bond
• sigma bond
The table below contains selected information about chlorine and two compounds containing chlorine. One piece of information is missing for each of the substances in the table.
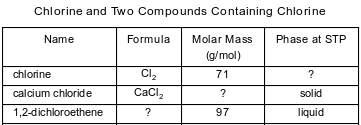
Explain, in terms of electrons, why the compound containing calcium and chlorine is classified as an ionic compound.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Electrons are transferred from the metal to the nonmetal.
• Calcium loses electrons and chlorine gains electrons.
• Electrons were transferred.
The formula below represents a molecule of butanamide.
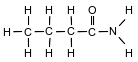
State the type of chemical bond between a hydrogen atom and the nitrogen atom in the molecule.
Allow 1 credit. Acceptable responses include, but are not limited to:
• polar covalent bond
• covalent
• polar covalent
Potassium phosphate, K3PO4, is a source of dietary potassium found in a popular cereal. According to the Nutrition-Facts label shown on the boxes of this brand of cereal, the accepted value for a one-cup serving of this cereal is 170. milligrams of potassium. The minimum daily requirement of potassium is 3500 milligrams for an adult human.
Identify two types of chemical bonding in the source of dietary potassium in this cereal.
Allow 1 credit. Acceptable responses include, but are not limited to:
• polar covalent and ionic
• ionic and covalent
• polar and ionic
