Topic: Chemical Bonds
Chemical Bonds
Which element has metallic bonds at room temperature?
(1) bromine
(2) cesium
(3) krypton
(4) sulfur
A solid sample of copper is an excellent conductor of electric current. Which type of chemical bonds are in the sample?
(1) ionic bonds
(2) metallic bonds
(3) nonpolar covalent bonds
(4) polar covalent bonds
The particle diagram below represents a solid sample of silver.

Which type of bonding is present when valence electrons move within the sample?
(1) metallic bonding
(2) hydrogen bonding
(3) covalent bonding
(4) ionic bonding
What is the number of electrons shared between the atoms in an I2 molecule?
(1) 7
(2) 2
(3) 8
(4) 4
What occurs when potassium reacts with chlorine to form potassium chloride?
(1) Electrons are shared and the bonding is ionic.
(2) Electrons are shared and the bonding is covalent.
(3) Electrons are transferred and the bonding is ionic.
(4) Electrons are transferred and the bonding is covalent.
Which type of bonding is present in a sample of an element that is malleable?
(1) ionic
(2) metallic
(3) nonpolar covalent
(4) polar covalent
Which atoms will bond when valence electrons are transferred from one atom to the other?
(1) O and Se
(2) O and Sr
(3) O and H
(4) O and P
At STP, which substance has metallic bonding?
(1) ammonium chloride
(2) barium oxide
(3) iodine
(4) silver
Given the formula for hydrazine:
![]()
How many pairs of electrons are shared between the two nitrogen atoms?
(1) 1
(2) 2
(3) 3
(4) 4
An ionic bond can be formed when one or more electrons are
(1) equally shared by two atoms
(2) unequally shared by two atoms
(3) transferred from the nucleus of one atom to the nucleus of another atom
(4) transferred from the valence shell of one atom to the valence shell of another atom
Which statement describes the energy and bonding changes as two atoms of fluorine become a molecule of fluorine?
(1) Energy is absorbed as a bond is broken.
(2) Energy is absorbed as a bond is formed.
(3) Energy is released as a bond is broken.
(4) Energy is released as a bond is formed.
Given the equation representing a reaction:
H + H → H2
What occurs during this reaction?
(1) A bond is broken and energy is absorbed.
(2) A bond is broken and energy is released.
(3) A bond is formed and energy is absorbed.
(4) A bond is formed and energy is released.
Fatty acids, a class of compounds found in living things, are organic acids with long hydrocarbon chains. Linoleic acid, an unsaturated fatty acid, is essential for human skin flexibility and smoothness. The formula below represents a molecule of linoleic acid.
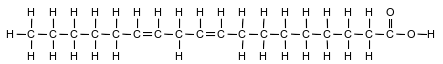
Identify the type of chemical bond between the oxygen atom and the hydrogen atom in the linoleic acid molecule.
Allow 1 credit. Acceptable responses include, but are not limited to:
• covalent
• polar covalent
• polar covalent bond
• sigma bond
The table below contains selected information about chlorine and two compounds containing chlorine. One piece of information is missing for each of the substances in the table.
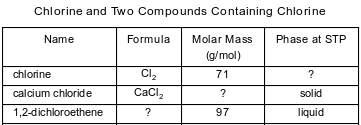
Explain, in terms of electrons, why the compound containing calcium and chlorine is classified as an ionic compound.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Electrons are transferred from the metal to the nonmetal.
• Calcium loses electrons and chlorine gains electrons.
• Electrons were transferred.
The formula below represents a molecule of butanamide.
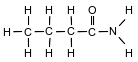
State the type of chemical bond between a hydrogen atom and the nitrogen atom in the molecule.
Allow 1 credit. Acceptable responses include, but are not limited to:
• polar covalent bond
• covalent
• polar covalent
