Topic: Excited States Bright Line Spectrum
Excited States Bright Line Spectrum
Which electron configuration could represent the electrons in a sodium atom in an excited state?
(1) 2-8
(2) 2-8-1
(3) 2-7-1
(4) 2-7-2
Which electron confi guration represents the electrons in an atom of sulfur in an excited state?
(1) 2 – 8 – 6
(2) 2 – 7 – 7
(3) 2 – 8 – 7
(4) 2 – 7 – 8
Which electron confi guration represents an atom of chlorine in an excited state?
(1) 2-8-7-2
(2) 2-8-7
(3) 2-8-8
(4) 2-7-8
Which electron configuration represents the electrons in an atom of sodium in the ground state at STP?
(1) 2-8-1
(2) 2-7-2
(3) 2-8-6
(4) 2-7-7
Which electron configuration represents the electrons of an atom in an excited state?
(1) 2-5
(2) 2-8-5
(3) 2-5-1
(4) 2-6
Which electron configuration represents a potassium atom in an excited state?
(1) 2-7-6
(2) 2-8-5
(3) 2-8-8-1
(4) 2-8-7-2
Which electron configuration represents the electrons of an atom in an excited state?
(1) 2–2
(2) 2–2–1
(3) 2–8
(4) 2–8–1
Which electron configuration represents the electrons in an atom of calcium in an excited state?
(1) 2–8–8
(2) 2–8–8–2
(3) 2–7–8–1
(4) 2–7–8–3
Which electron configuration represents the electrons of an atom of neon in an excited state?
(1) 2-7
(2) 2-8
(3) 2-7-1
(4) 2-8-1
Which electron configuration represents the distribution of electrons in a potassium atom in the ground state?
(1) 2-8-8-1
(2) 2-8-7-2
(3) 2-8-5
(4) 2-7-6
Which electron configuration represents an excited state for an atom of calcium?
(1) 2-8-7-1
(2) 2-8-7-2
(3) 2-8-7-3
(4) 2-8-8-2
Which electron configuration represents an atom of chlorine in an excited state?
(1) 2-7-7
(2) 2-7-8
(3) 2-8-7
(4) 2-8-8
Which electron configuration represents an atom of magnesium in an excited state?
(1) 2–7–3
(2) 2–7–6
(3) 2–8–2
(4) 2–8–5
Fireworks that contain metallic salts such as sodium, strontium, and barium can generate bright colors. A technician investigates what colors are produced by the metallic salts by performing flame tests. During a flame test, a metallic salt is heated in the flame of a gas burner. Each metallic salt emits a characteristic colored light in the flame.
Explain why the electron configuration of 2-7-1-1 represents a sodium atom in an excited state.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The configuration represents a higher energy state than sodium’s ground state, 2-8-1.
• Not all 11 electrons are in their lowest possible energy levels.
• A second shell electron has moved to the fourth shell.
• A lower shell electron is shown in a higher shell.
The Bohr model of the atom was developed in the early part of the twentieth century. A diagram of the Bohr model for one atom, in the ground state, of a specific element is shown below. The nucleus of this atom contains 4 protons and 5 neutrons.
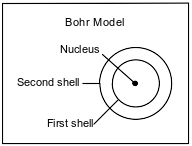
State the number of electrons in each shell in this atom in the ground state.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Number of electrons in first shell: 2 or 2e−
• Number of electrons in second shell: 2 or 2e−
