Topic: Formula Mass And Gram Formula Mass
Formula Mass And Gram Formula Mass
Which quantity is equal to one mole of Au?
(1) the atomic mass in grams
(2) the atomic number in grams
(3) the mass of neutrons in grams
(4) the number of neutrons in grams
What is the mass of 1.5 moles of CO2?
(1) 66 g
(2) 44 g
(3) 33 g
(4) 29 g
What is the gram-formula mass of Ca(OH)2?
(1) 29 g/mol
(2) 54 g/mol
(3) 57 g/mol
(4) 74 g/mol
What is the number of moles of KF in a 29-gram sample of the compound?
(1) 1.0 mol
(2) 2.0 mol
(3) 0.50 mol
(4) 5.0 mol
Vitamin C, also known as ascorbic acid, is water soluble and cannot be produced by the human body. Each day, a person’s diet should include a source of vitamin C, such as orange juice. Ascorbic acid has a molecular formula of C6H8O6 and a gram-formula mass of 176 grams per mole.
Determine the number of moles of vitamin C in an orange that contains 0.071 gram of vitamin C.
Allow 1 credit. Significant figures do not need to be shown. Acceptable responses include, but are
• not limited to:
• 4.0 × 10−4 mol
• 0.000 40 mol
The equation below represents the reaction between 1-butene and bromine to form the compound 1,2-dibromobutane, C4H8Br2.

Determine the gram-formula mass of 1-butene.
Allow 1 credit for 56 g/mol. Significant figures do not need to be shown.
The element boron, a trace element in Earth’s crust, is found in foods produced from plants. Boron has only two naturally occurring stable isotopes, boron-10 and boron-11.
One sample of a green vegetable contains 0.0035 gram of boron. Determine the total number of moles of boron in this sample.
Allow 1 credit for 0.000 32 mol or 3.2 × 10−4 mol. Significant figures do not need to be shown.
Ethene and hydrogen can react at a faster rate in the presence of the catalyst platinum. The equation below represents a reaction between ethene and hydrogen.
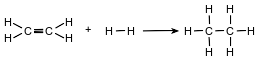
Determine the molar mass of the product.
Allow 1 credit for 30 g/mol, 30. g/mol, or for any value from 30.06 g/mol to 30.1 g/mol, inclusive.
The balanced equation below represents the reaction of glucose, C6H12O6, with oxygen at 298 K and 101.3 kPa.
C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(ℓ)
Determine the mass of CO2 produced when 9.0 grams of glucose completely reacts with 9.6 grams of oxygen to produce 5.4 grams of water.
Allow 1 credit for 13.2 g or for any value from 13.155 g to 13.2042 g, inclusive.
A student made a copper bracelet by hammering a small copper bar into the desired shape. The bracelet has a mass of 30.1 grams and was at a temperature of 21°C in the classroom. After the student wore the bracelet, the bracelet reached a temperature of 33°C. Later, the student removed the bracelet and placed it on a desk at home, where it cooled from 33°C to 19°C. The specific heat capacity of copper is 0.385 J/g•K.
Determine the number of moles of copper in the bracelet.
Allow 1 credit for 0.474 mol or for any value from 0.47 mol to 0.47402 mol, inclusive, or for 0.5 mol.
A sample of calcium carbonate, CaCO3, has a mass of 42.2 grams. Calcium carbonate has a gram-formula mass of 100. g/mol.
Show a numerical setup for calculating the number of moles in the sample of CaCO3.
Allow 1 credit. Acceptable responses include, but are not limited to:
• chem12017-rg_g4.png
The reaction between aluminum and an aqueous solution of copper(II) sulfate is represented by the unbalanced equation below.
Al(s) + CuSO4(aq) → Al2(SO4)3(aq) + Cu(s)
Determine the total mass of Cu produced when 1.08 grams of Al reacts completely with 9.58 grams of CuSO4 to produce 6.85 grams of Al2(SO4)3.
Allow 1 credit for 3.81 g.
A total of 1.4 moles of sodium nitrate is dissolved in enough water to make 2.0 liters of an aqueous solution. The gram-formula mass of sodium nitrate is 85 grams per mole.
Show a numerical setup for calculating the mass of the solute used to make the solution.
Allow 1 credit. Acceptable responses include, but are not limited to:
• (85 g/mol)(1.4 mol)
• (1.4)(85)
A student constructs an electrochemical cell during a laboratory investigation. When the switch is closed, electrons flow through the external circuit. The diagram and equation below represent this cell and the reaction that occurs.

Determine the number of moles of Al(s) needed to completely react with 9.0 moles of Ni2+(aq) ions.
Allow 1 credit for 6.0 mol. Significant figures do not need to be shown.
Paintball is a popular recreational activity that uses a metal tank of compressed carbon dioxide or nitrogen to launch small capsules of paint. A typical tank has a volume of 508 cubic centimeters. A 340.-gram sample of carbon dioxide is added to the tank before it is used for paintball. At 20.°C, this tank contains both CO2(g) and CO2(ℓ). After a paintball game, the tank contains only CO2(g).
Determine the total number of moles of CO2 added to the tank before it is used for paintball.
Allow 1 credit for 7.73 mol or for any value from 7.7 mol to 8 mol, inclusive.
