Topic: Functional Groups And Types Of Organic Compounds
Functional Groups And Types Of Organic Compounds
Given the organic functional group:
![]()
Which class of organic compounds has molecules with this functional group?
(1) aldehydes
(2) esters
(3) ketones
(4) organic acids
Given the formula for a compound:
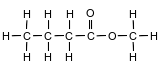
What is the name of this compound?
(1) methyl butanoate
(2) methyl butyl ether
(3) pentanone
(4) pentanoic acid
Which functional group contains a nitrogen atom and an oxygen atom?
(1) ester
(2) ether
(3) amide
(4) amine
An alcohol and an ether have the same molecular formula, C2H6O. These two compounds have
(1) the same functional group and the same physical and chemical properties
(2) the same functional group and different physical and chemical properties
(3) different functional groups and the same physical and chemical properties
(4) different functional groups and different physical and chemical properties
Amines, amides, and amino acids are categories of
(1) isomers
(2) isotopes
(3) organic compounds
(4) inorganic compounds
Ethyl ethanoate is used as a solvent for varnishes and in the manufacture of artifi cial leather. The formula below represents a molecule of ethyl ethanoate.
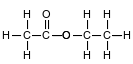
Write the name of the class of organic compounds to which this compound belongs.
Allow 1 credit for ester or esters.
Draw a structural formula for methanal.
Allow 1 credit.
• Examples of 1-credit responses:
• ![]()
• ![]()
• ![]()
Fatty acids, a class of compounds found in living things, are organic acids with long hydrocarbon chains. Linoleic acid, an unsaturated fatty acid, is essential for human skin flexibility and smoothness. The formula below represents a molecule of linoleic acid.
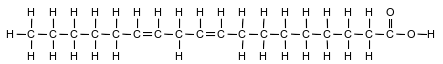
On the diagram in your answer booklet, circle the organic acid functional group.
Allow 1 credit.
• Examples of 1-credit reponses:
• 
Methanol can be manufactured by a reaction that is reversible. In the reaction, carbon monoxide gas and hydrogen gas react using a catalyst. The equation below represents this system at equilibrium.
CO(g) + 2H2(g) ⇌ CH3OH(g) + energy
State the class of organic compounds to which the product of the forward reaction belongs.
Allow 1 credit for alcohol or alcohols.
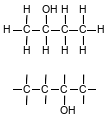
Draw a structural formula for 2-butanol.
Allow 1 credit.
• Examples of 1-credit responses
• 
Polyvinyl chloride (PVC) is a polymer used to make drain pipes, flooring, electric wire insulation, and some plastic bottles. Making PVC requires several reactions. The first step is represented by the equation below.
![]()
The 1,2-dichloroethane is converted to vinyl chloride. To produce PVC, the vinyl chloride monomer is polymerized, as represented by the equation below.
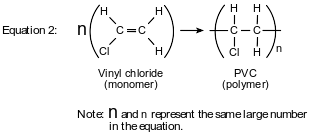
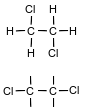
Draw a structural formula for the product of equation 1.
Allow 1 credit.
• Examples of 1-credit responses:
• 
The equation below represents the reaction between 2-methylpropene and hydrogen chloride gas.
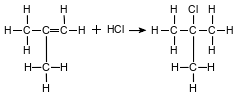
Identify the class of organic compounds to which the product belongs.
Allow 1 credit. Acceptable responses include, but are not limited to:
• halide
• halocarbons
• alkyl halide
Molecules containing two carbon atoms and a functional group have many home and industrial uses. These compounds can be produced by a variety of reactions, as shown by the equations below.
Equation 1: C2H4 + H2O → CH3CH2OH
Equation 2: 2CH3CH2OH + O2 → 2CH3CHO + 2H2O
Equation 3: 2CH3CHO + O2 → 2CH3COOH
Identify the class of organic compounds to which the product in equation 3 belongs.
Allow 1 credit. Acceptable responses include, but are not limited to:
• organic acid
• carboxylic acid
• acids
In industry, ethanol is primarily produced by two different reactions. One process involves the reaction of glucose in the presence of an enzyme that acts as a catalyst. The equation below represents this reaction.
![]()
In another reaction, ethanol is produced from ethene and water. The equation below represents this reaction in which H2SO4 is a catalyst.
![]()
Industrial ethanol can be oxidized using a catalyst to produce ethanal. The equation representing this oxidation is shown below.
![]()
Draw a structural formula for the organic product in equation 3.
Allow 1 credit.
• Examples of 1-credit responses:
• 
The equation below represents a reaction between propene and hydrogen bromide.
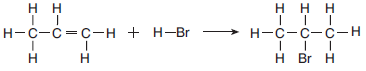
Cyclopropane, an isomer of propene, has a boiling point of –33ºC at standard pressure and is represented by the formula below.
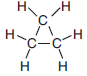
Identify the class of organic compounds to which the product of this reaction belongs.
Allow 1 credit. Acceptable responses include, but are not limited to:
• halide
• halocarbon
• alkyl halide
