Topic: Identification Of Element
Identification Of Element
What is the total number of neutrons in an atom of K-42?
(1) 19
(2) 20
(3) 23
(4) 42
Compared to an atom of C-12, an atom of C-14 has a greater
(1) number of electrons
(2) number of protons
(3) atomic number
(4) mass number
Which two notations represent isotopes of the same element?
(1) 147N and 187N
(2) 207N and 2010Ne
(3) 147N and 1710Ne
(4) 197N and 1610Ne
Which notations represent hydrogen isotopes?
(1) 11H and 21H
(2) 11H and 42H
(3) 12H and 13H
(4) 21H and 72H
Which particle has two neutrons?
(1) 10n
(2) 11H
(3) 21H
(4) 42He
Each diagram below represents the nucleus of an atom.
![]()
How many different elements are represented by the diagrams?
(1) 1
(2) 2
(3) 3
(4) 4
All atoms of uranium have the same
(1) mass number
(2) atomic number
(3) number of neutrons plus protons
(4) number of neutrons plus electrons
All phosphorus atoms have the same
(1) atomic number
(2) mass number
(3) number of neutrons plus the number of electrons
(4) number of neutrons plus the number of protons
Which notations represent different isotopes of the element sodium?
(1) 32S and 34S
(2) S2− and S6+
(3) Na+ and Na0
(4) 22Na and 23Na
The four naturally occurring isotopes of sulfur are S-32, S-33, S-34, and S-36. The table below shows the atomic mass and percent natural abundance for these isotopes.
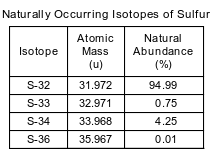
State both the number of protons and the number of neutrons in an S-33 atom.
Allow 1 credit for 16 protons and 17 neutrons.
The only naturally occurring isotopes of nitrogen are N-14 and N-15.
State the number of protons in an atom of N-15.
Allow 1 credit for 7 or seven.
When uranium-235 nuclei are bombarded with neutrons, many different combinations of smaller nuclei can be produced. The production of neodymium-150 and germanium-81 in one of these reactions is represented by the equation below.
![]()
Germanium-81 and uranium-235 have different decay modes. Ge-81 emits beta particles and has a half-life of 7.6 seconds.
State the number of protons and number of neutrons in a neodymium-150 atom.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Protons: 60
• Neutrons: 90
The isotope Rn-222 is produced by the decay of uranium in Earth’s crust. Some of this isotope leaks into basements of homes in areas where the ground is more porous. An atom of Rn-222 decays to an atom of Pb-206 through a series of steps as shown on the graph below.
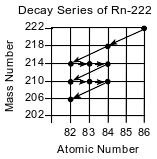
Determine the number of neutrons in an atom of Pb-214.
Allow 1 credit for 132.
The diagrams below represent four different atomic nuclei.
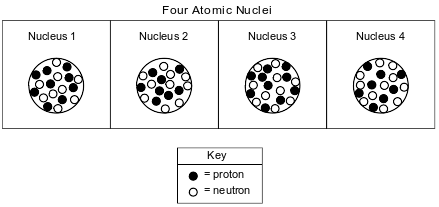
Determine the mass number of the nuclide represented by nucleus 2.
Allow 1 credit for 18.
The radioisotope Mo-99 naturally decays to produce the metastable isotope Tc-99m, which is used in medical diagnosis. A doctor can obtain images of organs and bones by injecting a patient with a solution of Tc-99m. The half-life of the metastable Tc-99m is six hours.
State both the number of protons and the number of neutrons in a Tc-99 nuclide.
Allow 1 credit for 43 protons and 56 neutrons.
