Topic: Kinetics Equilibrium
Kinetics Equilibrium
Hydrochloric acid reacts faster with powdered zinc than with an equal mass of zinc strips because the greater surface area of the powdered zinc
(1) decreases the frequency of particle collisions
(2) decreases the activation energy of the reaction
(3) increases the frequency of particle collisions
(4) increases the activation energy of the reaction
According to which theory or law is a chemical reaction most likely to occur when two particles with the proper energy and orientation interact with each other?
(1) atomic theory
(2) collision theory
(3) combined gas law
(4) law of conservation of matter
The equation below represents a reaction between two molecules, X2 and Z2. These molecules form an “activated complex,” which then forms molecules of the product.
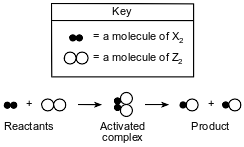
Which diagram represents the most likely orientation of X2 and Z2 when the molecules collide with proper energy, producing an activated complex?
(1) ![]()
(2) ![]()
(3) ![]()
(4) ![]()
A chemical reaction occurs when reactant particles
(1) are separated by great distances
(2) have no attractive forces between them
(3) collide with proper energy and proper orientation
(4) convert chemical energy into nuclear energy
Given the balanced equation representing a reaction:
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) + energy
Which change in reaction conditions will increase the frequency of effective collisions between reactant molecules?
(1) decreasing the pressure of the reactants
(2) decreasing the temperature of the reactants
(3) increasing the concentration of the reactants
(4) increasing the volume of the reactants
A chemical reaction is most likely to occur when the colliding particles have the proper
(1) energy and orientation
(2) solubility and density
(3) ionic radii and mass
(4) atomic radii and volume
A reaction is most likely to occur when the colliding particles have proper orientation and
(1) mass
(2) volume
(3) half-life
(4) energy
As the temperature of a reaction increases, it is expected that the reacting particles collide
(1) more often and with greater force
(2) more often and with less force
(3) less often and with greater force
(4) less often and with less force
The collision theory states that a reaction is most likely to occur when the reactant particles collide with the proper
(1) formula masses
(2) molecular masses
(3) density and volume
(4) energy and orientation
A reaction will most likely occur if the colliding particles have the proper
(1) mass, only
(2) mass and volume
(3) orientation, only
(4) orientation and energy
Which statement explains why increasing the temperature increases the rate of a chemical reaction, while other conditions remain the same?
(1) The reacting particles have less energy and collide less frequently.
(2) The reacting particles have less energy and collide more frequently.
(3) The reacting particles have more energy and collide less frequently.
(4) The reacting particles have more energy and collide more frequently.
Explain, in terms of collisions, why increasing the surface area of the hot carbon increases the rate of the forward reaction.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Increasing the surface area of the hot carbon increases the frequency of effective collisions, which increases the rate of the forward reaction.
• More collisions between C atoms and H2O molecules speed up the reaction.
• More effective collisions occur.
Methanol can be manufactured by a reaction that is reversible. In the reaction, carbon monoxide gas and hydrogen gas react using a catalyst. The equation below represents this system at equilibrium.
CO(g) + 2H2(g) ⇌ CH3OH(g) + energy
Explain, in terms of collision theory, why increasing the concentration of H2(g) in this system will increase the concentration of CH3OH(g).
Allow 1 credit. Acceptable responses include, but are not limited to:
• More H2(g) molecules collide with CO(g) molecules, producing more CH3OH(g).
• Adding H2 increases the number of effective collisions to produce more methanol.
• A greater number of effective collisions occur.
Millions of tons of ammonia are produced each year for use as fertilizer to increase food production. Most of the hydrogen needed to produce ammonia comes from methane gas reacting with steam. This reaction, which occurs in a container under controlled conditions, is shown below in unbalanced equation 1.
Equation 1: CH4(g) + H2O(g) + energy → CO(g) + H2(g)
The reaction that produces ammonia is represented by balanced equation 2, shown below. A catalyst can be used to increase the rate of the reaction.
Equation 2: N2(g) + 3H2(g) → 2NH3(g) + energy
A potential energy diagram for equation 2 is shown below.
Explain, in terms of collision theory, why an increase in temperature increases the rate of reaction between methane gas and steam.
Allow 1 credit. Acceptable responses include, but are not limited to:
• An increase in temperature causes a greater number of effective collisions between methane and water molecules to occur.
• A greater number of collisions per second make the reaction rate faster.
• More molecules collide with sufficient energy.
Carbon monoxide, CO(g), is a toxic gas found in automobile exhaust. The concentration of CO(g) can be decreased by using a catalyst in the reaction between CO(g) and O2(g). This reaction is represented by the balanced equation below.
![]()
Explain, in terms of collision theory, why an increase in temperature increases the rate of the reaction.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The rate of the chemical reaction increases because the reactant molecules move faster and collide with more kinetic energy.
• Increasing the temperature causes more frequent collisions.
• As molecules acquire more kinetic energy, the probability of effective collisions increases.
• More reactant molecules collide with sufficient energy.
