Topic: Nuclear Energy
Nuclear Energy
Given the equation representing a reaction:
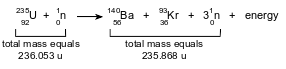
Which statement explains the energy term in this reaction?
(1) Mass is gained due to the conversion of mass to energy.
(2) Mass is gained due to the conversion of energy to mass.
(3) Mass is lost due to the conversion of mass to energy.
(4) Mass is lost due to the conversion of energy to mass.
The energy released by a nuclear fusion reaction is produced when
(1) energy is converted to mass
(2) mass is converted to energy
(3) heat is converted to temperature
(4) temperature is converted to heat
The energy released during a nuclear reaction is a result of
(1) breaking chemical bonds
(2) forming chemical bonds
(3) mass being converted to energy
(4) energy being converted to mass
Which process converts mass into energy?
(1) distillation of ethanol
(2) filtration of a mixture
(3) fusion of hydrogen atoms
(4) ionization of cesium atoms
Which change occurs during a nuclear fission reaction?
(1) Covalent bonds are converted to ionic bonds.
(2) Isotopes are converted to isomers.
(3) Temperature is converted to mass.
(4) Matter is converted to energy.
Which net change occurs in a nuclear fusion reaction?
(1) Ionic bonds are broken.
(2) Ionic bonds are formed.
(3) Energy is converted to mass.
(4) Mass is converted to energy.
Given the equation representing a reaction where the masses are expressed in atomic mass units:
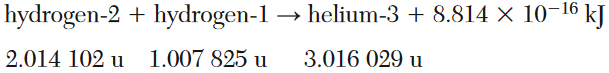
Which phrase describes this reaction?
(1) a chemical reaction and mass being converted to energy
(2) a chemical reaction and energy being converted to mass
(3) a nuclear reaction and mass being converted to energy
(4) a nuclear reaction and energy being converted to mass
During a nuclear reaction, mass is converted into
(1) charge
(2) energy
(3) isomers
(4) volume
Given the balanced equation representing a nuclear reaction:
![]()
Which phrase identifies and describes this reaction?
(1) fission, mass converted to energy
(2) fission, energy converted to mass
(3) fusion, mass converted to energy
(4) fusion, energy converted to mass
Which statement describes a benefit of using fission reactions?
(1) Radioactive waste must be stored for long periods of time.
(2) Nuclear fuel consists of stable isotopes.
(3) Gamma radiation is produced.
(4) Large amounts of energy are produced per mole of reactant.
Using equal masses of reactants, which statement describes the relative amounts of energy released during a chemical reaction and a nuclear reaction?
(1) The chemical and nuclear reactions release equal amounts of energy.
(2) The nuclear reaction releases half the amount of energy of the chemical reaction.
(3) The chemical reaction releases more energy than the nuclear reaction.
(4) The nuclear reaction releases more energy than the chemical reaction.
Compared to the energy released per mole of reactant during chemical reactions, the energy released per mole of reactant during nuclear reactions is
(1) much less
(2) much greater
(3) slightly less
(4) slightly greater
Given two balanced equations, each representing a reaction:
Equation 1: 22688Ra →22286Rn + 42He + 4.8 × 108 kJ
Equation 2: C3H8 + 5O2 → 3CO2 + 4H2O + 2.2 × 103 kJ
Which statement compares the energy terms in these two equations?
(1) Equation 1 shows 2.2 × 105 times more energy being absorbed.
(2) Equation 2 shows 2.2 × 105 times more energy being absorbed.
(3) Equation 1 shows 2.2 × 105 times more energy being released.
(4) Equation 2 shows 2.2 × 105 times more energy being released.
Which reaction releases the greatest amount of energy per mole of reactant?
(1) decomposition
(2) esterification
(3) fermentation
(4) fission
A breeder reactor is one type of nuclear reactor. In a breeder reactor, uranium-238 is transformed in a series of nuclear reactions into plutonium-239.
The plutonium-239 can undergo fission as shown in the equation below. The X represents a missing product in the equation.
![]()
Compare the amount of energy released by 1 mole of completely fissioned plutonium-239 to the amount of energy released by the complete combustion of 1 mole of methane.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The fission of one mole of Pu-239 releases much more energy than the combustion of one mole of CH4.
• The energy released during the chemical reaction is less than the energy released during the nuclear reaction.
• greater for 23994Pu
