Topic: Octet Rule
Octet Rule
In which group on the Periodic Table would a nonmetallic element belong if atoms of this element tend to gain two electrons to complete their valence shell?
(1) 14
(2) 15
(3) 16
(4) 17
In the formula for the compound XCl4, the X could represent
(1) C
(2) H
(3) Mg
(4) Zn
In the formula XSO4, the symbol X could represent the element
(1) Al
(2) Ar
(3) Mg
(4) Na
Which group on the Periodic Table has elements with atoms that tend not to bond with atoms of other elements?
(1) Group 1
(2) Group 2
(3) Group 17
(4) Group 18
Which atom attains a stable valence electron configuration by bonding with another atom?
(1) neon
(2) radon
(3) helium
(4) hydrogen
Which statement explains why neon is a Group 18 element?
(1) Neon is a gas at STP.
(2) Neon has a low melting point.
(3) Neon atoms have a stable valence electron configuration.
(4) Neon atoms have two electrons in the first shell.
Which atom in the ground state has the same electron configuration as a calcium ion, Ca2+, in the ground state?
(1) Ar
(2) K
(3) Mg
(4) Ne
Identify the element in Period 3 that is an unreactive gas at STP.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Ar
• argon
• element 18
A thiol is very similar to an alcohol, but a thiol has a sulfur atom instead of an oxygen atom in the functional group. The equation below represents a reaction of methanethiol and iodine, producing dimethyl disulfide and hydrogen iodide.
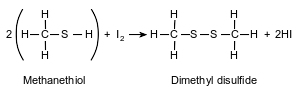
Explain, in terms of electron configuration, why sulfur atoms and oxygen atoms form compounds with similar molecular structures.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Sulfur and oxygen atoms both have 6 valence electrons.
• Atoms of both elements need the same number of electrons to complete their outer shells.
Three elements, represented by D, E, and Q, are located in Period 3. Some properties of these elements are listed in the table below. A student’s experimental result indicates that the density of element Q is 2.10 g/cm3, at room temperature and standard pressure.
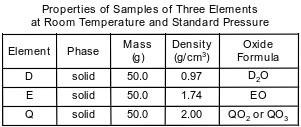
Identify the group on the Periodic Table to which element D belongs.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Group 1
• alkali metals
In the late 1800s, Dmitri Mendeleev developed a periodic table of the elements known at that time. Based on the pattern in his periodic table, he was able to predict properties of some elements that had not yet been discovered. Information about two of these elements is shown in the table below.
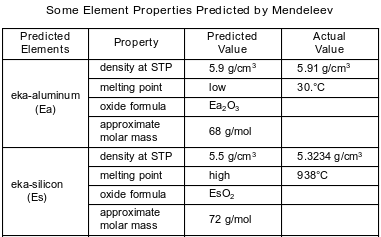
Write a chemical formula for the compound formed between Ea and Cl.
Allow 1 credit. Acceptable responses include, but are not limited to:
• EaCl3
• GaCl3
Silver-plated utensils were popular before stainless steel became widely used to make eating utensils. Silver tarnishes when it comes in contact with hydrogen sulfide, H2S, which is found in the air and in some foods. However, stainless steel does not tarnish when it comes in contact with hydrogen sulfide.
In the ground state, an atom of which noble gas has the same electron configuration as the sulfide ion in Ag2S?
Allow 1 credit. Acceptable responses include, but are not limited to:
• Ar
• argon
• element 18
The Lewis electron-dot diagrams for three substances are shown below.
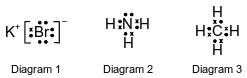
Identify the noble gas that has atoms with the same electron configuration as the positive ion represented in diagram 1, when both the atoms and the ion are in the ground state.
Allow 1 credit. Acceptable responses include, but are not limited to:
• argon
• Ar
The formulas and names of four chloride compounds are shown in the table below.
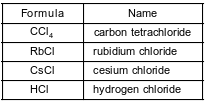
Identify the noble gas that has atoms with the same electron configuration as the metal ions in rubidium chloride, when both the atoms and the ions are in the ground state.
Allow 1 credit for Kr or krypton.
When magnesium is ignited in air, the magnesium reacts with oxygen and nitrogen. The reaction between magnesium and nitrogen is represented by the unbalanced equation below.
Mg(s) + N2(g) → Mg3N2(s)
In the ground state, which noble gas has atoms with the same electron configuration as a magnesium ion?
Allow 1 credit for Ne or neon.
