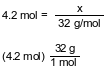Topic: Percent Composition
Percent Composition
What is the percent composition by mass of nitrogen in the compound N2H4 (gram-formula mass = 32 g/mol)?
(1) 13%
(2) 44%
(3) 88%
(4) 93%
What is the number of moles of CO2 in a 220.-gram sample of CO2 (gram-formula mass = 44 g/mol)?
(1) 0.20 mol
(2) 5.0 mol
(3) 15 mol
(4) 44 mol
What is the number of moles of KF in a 29-gram sample of the compound?
(1) 1.0 mol
(2) 2.0 mol
(3) 0.50 mol
(4) 5.0 mol
What is the percent composition by mass of nitrogen in (NH4)2CO3 (gram-formula mass = 96.0 g/mol)?
(1) 14.6%
(2) 29.2%
(3) 58.4%
(4) 87.5%
A metal worker uses a cutting torch that operates by reacting acetylene gas, C2H2(g), with oxygen gas, O2(g), as shown in the unbalanced equation below.
C2H2(g) + O2(g) → CO2(g) + H2O(g) + heat
Determine the mass of 25 moles of acetylene (gram-formula mass = 26 g/mol).
Allow 1 credit for 650 g or any value from 650 g to 651 g, inclusive.
During a laboratory activity, appropriate safety equipment is used and safety procedures are followed. A student separates a sample of rock salt that has two components; NaCl(s) and small insoluble rock particles. First, the student thoroughly stirs the sample of rock salt into a sample of water in a fl ask. The mixture in the fl ask is fi ltered using the lab apparatus shown below.
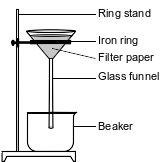
The water is evaporated from the beaker. The fi lter paper and its contents are dried. The data collected by the student are shown in the table below.
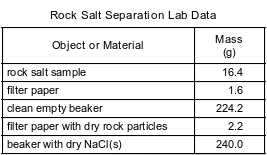
Show a numerical setup for calculating the percent by mass of NaCl in the rock salt sample.
Allow 1 credit. Acceptable responses include, but are not limited to:
• ![]()
• ![]()
• ![]()
• ![]()
The enclosed cabin of a submarine has a volume of 2.4 × 105 liters, a temperature of 312 K, and a pressure of 116 kPa. As people in the cabin breathe, carbon dioxide gas, CO2(g), can build up to unsafe levels. Air in the cabin becomes unsafe to breathe when the mass of CO2(g) in this cabin exceeds 2156 grams.
Determine the number of moles of CO2(g) in the submarine cabin at which the air becomes unsafe to breathe. The gram-formula mass of CO2 is 44.0 g/mol.
Allow 1 credit for 49.0 mol or any value from 49 mol to 50. mol, inclusive.
A hydrate is a compound that has water molecules within its crystal structure. Magnesium sulfate heptahydrate, MgSO4•7H2O, is a hydrated form of magnesium sulfate. The hydrated compound has 7 moles of H2O for each mole of MgSO4. When 5.06 grams of MgSO4•7H2O are heated to at least 300.°C in a crucible by using a laboratory burner, the water molecules are released. The sample was heated repeatedly, until the remaining MgSO4 had a constant mass of 2.47 grams. During this laboratory activity, appropriate safety equipment was used and safety procedures were followed.
Using the lab data, show a numerical setup for calculating the percent composition by mass of water in the hydrated compound.
Allow 1 credit. Acceptable responses include, but are not limited to:
• ![]()
During a laboratory activity, appropriate safety equipment was used and safety procedures were followed. A laboratory technician heated a sample of solid KClO3 in a crucible to determine the percent composition by mass of oxygen in the compound. The unbalanced equation and the data for the decomposition of solid KClO3 are shown below.
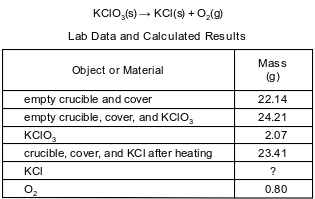
Based on the lab data, show a numerical setup to determine the number of moles of O2 produced. Use 32 g/mol as the gram-formula mass of O2.
Allow 1 credit. Acceptable responses include, but are not limited to:
• 
Show a numerical setup for calculating the percent composition by mass of oxygen in Al2O3 (gram-formula mass = 102 g/mol).
Allow 1 credit. Acceptable responses include, but are not limited to:
• 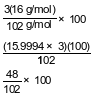
The diagram below represents a cylinder with a moveable piston containing 16.0 g of O2(g). At 298 K and 0.500 atm, the O2(g) has a volume of 24.5 liters.
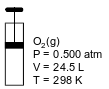
Determine the number of moles of O2(g) in the cylinder. The gram-formula mass of O2(g) is 32.0 g/mol.
Allow 1 credit for 0.500 mol or any value from 0.500 mol to 0.501 mol, inclusive.
Some compounds of silver are listed with their chemical formulas in the table below.
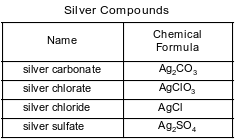
Show a numerical setup for calculating the percent composition by mass of silver in silver carbonate (gram-formula mass = 276 g/mol).
Allow 1 credit. Acceptable responses include, but are not limited to:
• 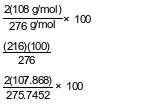
Given the unbalanced equation showing the reactants and product of a reaction occurring at 298 K and 100. kPa:
P4(s) + Cl2(g) → PCl3(ℓ) + energy
Show a numerical setup for calculating the percent composition by mass of chlorine in PCl3(ℓ) (gram-formula mass = 137 g/mol).
Allow 1 credit. Acceptable responses include, but are not limited to:
• 
Wood is mainly cellulose, a polymer produced by plants. One use of wood is as a fuel in campfires, fireplaces, and wood furnaces. The molecules of cellulose are long chains of repeating units. Each unit of the chain can be represented as C6H10O5. The balanced equation below represents a reaction that occurs when C6H10O5 is burned in air.
C6H10O5 + 6O2 → 6CO2 + 5H2O + heat
Show a numerical setup for calculating the percent composition by mass of carbon in C6H10O5 (gram-formula mass = 162.1 g/mol).
Allow 1 credit. Acceptable responses include, but are not limited to:
• 
Ammonia, NH3(g), can be used as a substitute for fossil fuels in some internal combustion engines. The reaction between ammonia and oxygen in an engine is represented by the unbalanced equation below.
NH3(g) + O2(g) → N2(g) + H2O(g) + energy
Show a numerical setup for calculating the mass, in grams, of a 4.2-mole sample of O2. Use 32 g/mol as the gram-formula mass of O2.
Allow 1 credit. Acceptable responses include, but are not limited to:
•