Topic: Rate Of A Chemical Reaction
Rate Of A Chemical Reaction
Which sample of HCl(aq) reacts at the fastest rate with a 1.0-gram sample of iron fi lings?
(1) 10. mL of 1 M HCl(aq) at 10.°C
(2) 10. mL of 1 M HCl(aq) at 25°C
(3) 10. mL of 3 M HCl(aq) at 10.°C
(4) 10. mL of 3 M HCl(aq) at 25°C
A 5.0-gram sample of Fe(s) is to be placed in 100. milliliters of HCl(aq). Which changes will result in the fastest rate of reaction?
(1) increasing the surface area of Fe(s) and increasing the concentration of HCl(aq)
(2) increasing the surface area of Fe(s) and decreasing the concentration of HCl(aq)
(3) decreasing the surface area of Fe(s) and increasing the concentration of HCl(aq)
(4) decreasing the surface area of Fe(s) and decreasing the concentration of HCl(aq)
Which factors have the greatest effect on the rate of a chemical reaction between AgNO3(aq) and Cu(s)?
(1) solution concentration and temperature
(2) solution concentration and pressure
(3) molar mass and temperature
(4) molar mass and pressure
For a reaction at equilibrium, which change can increase the rates of the forward and reverse reactions?
(1) a decrease in the concentration of the reactants
(2) a decrease in the surface area of the products
(3) an increase in the temperature of the system
(4) an increase in the activation energy of the forward reaction
The effect of a catalyst on a chemical reaction is to provide a new reaction pathway that results in a different
(1) potential energy of the products
(2) heat of reaction
(3) potential energy of the reactants
(4) activation energy
Addition of a catalyst can speed up a reaction by providing an alternate reaction pathway that has a
(1) lower activation energy
(2) higher activation energy
(3) lower heat of reaction
(4) higher heat of reaction
A catalyst increases the rate of a chemical reaction by
(1) providing an alternate reaction pathway
(2) providing the required heat of reaction
(3) increasing the potential energy of the products
(4) increasing the activation energy of the reaction
The balanced equation below represents the reaction between a 5.0-gram sample of zinc metal and a 0.5 M solution of hydrochloric acid. The reaction takes place in an open test tube at 298 K and 1 atm in a laboratory activity.
Zn(s) + 2HCl(aq) → H2(g) + ZnCl2(aq) + energy
State one change in reaction conditions, other than adding a catalyst, that will increase the rate of the reaction.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Increase the surface area of the zinc.
• Increase the temperature of the reaction.
• Use a more concentrated HCl(aq) solution.
Methanol can be manufactured by a reaction that is reversible. In the reaction, carbon monoxide gas and hydrogen gas react using a catalyst. The equation below represents this system at equilibrium.
CO(g) + 2H2(g) ⇌ CH3OH(g) + energy
State the effect on the rates of both the forward and reverse reactions if no catalyst is used in the system.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Rate of forward reaction: decreases/slower
• Rate of reverse reaction: decreases/slower
In a laboratory apparatus, a sample of lead(II) oxide reacts with hydrogen gas at high temperature. The products of this reaction are liquid lead and water vapor. As the reaction proceeds, water vapor and excess hydrogen gas leave the glass tube. The diagram and balanced equation below represent this reaction.
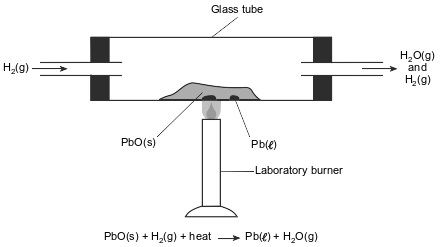
State one change in reaction conditions, other than adding a catalyst, that would cause the rate of this reaction to increase.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Increase the temperature.
• Increase the concentration of the hydrogen gas in the tube.
• Grind the metal oxide to increase its surface area.
The potential energy diagram and balanced equation shown below represent a reaction between solid carbon and hydrogen gas to produce 1 mole of C2H4(g) at 101.3 kPa and 298 K.
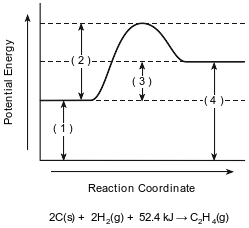
Identify one change in the reaction conditions, other than adding a catalyst, that can increase the rate of this reaction.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Increase the temperature.
• Increase the pressure.
• Increase the concentration of H2(g).
• Increase the surface area of the carbon.
Calcium reacts with water. This reaction is represented by the balanced equation below. The aqueous product of this reaction can be heated to evaporate the water, leaving a white solid, Ca(OH)2(s).
Ca(s) + 2H2O(ℓ) → Ca(OH)2(aq) + H2(g)
State one change in reaction conditions that will increase the rate of the reaction.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Increase the temperature of the water.
• Increase the surface area of Ca(s).
Automobile catalytic converters use a platinum catalyst to reduce air pollution by changing emissions such as carbon monoxide, CO(g), into carbon dioxide, CO2(g). The uncatalyzed reaction is represented by the balanced equation below.
2CO(g) + O2(g) → 2CO2(g) + heat
Compare the activation energy of the catalyzed reaction to the activation energy of the uncatalyzed reaction.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The catalyzed reaction has a lower activation energy than the uncatalyzed reaction.
• The activation energy is higher for the reaction with no catalyst.
Many breads are made by adding yeast to dough, causing the dough to rise. Yeast is a type of microorganism that produces the catalyst zymase, which converts glucose, C6H12O6, to ethanol and carbon dioxide gas. The balanced equation for this reaction is shown below.
![]()
Describe how the catalyst, zymase, speeds up this reaction.
Allow 1 credit. Acceptable responses include, but are not limited to:
• Zymase is a catalyst that provides an alternative pathway, which requires less energy.
• decreases the activation energy
• changes the reaction mechanism
Ethene and hydrogen can react at a faster rate in the presence of the catalyst platinum. The equation below represents a reaction between ethene and hydrogen.
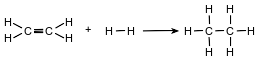
Explain, in terms of activation energy, why the catalyzed reaction occurs at a faster rate.
Allow 1 credit. Acceptable responses include, but are not limited to:
• The catalyzed reaction pathway has a lower activation energy than the original reaction.
• Less energy is needed.
